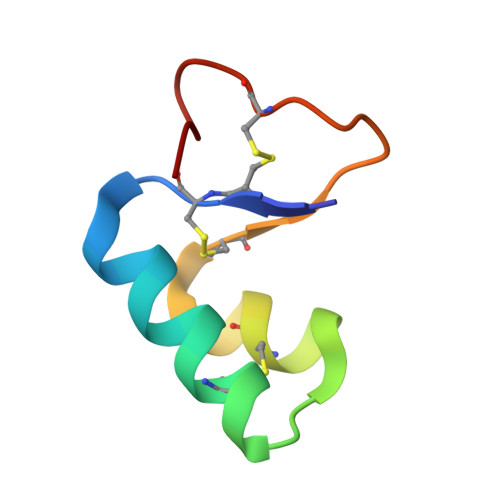
Structures of viscotoxins A1 and B2 from European mistletoe solved using native data alone.
Pal, A., Debreczeni, J.E., Sevvana, M., Gruene, T., Kahle, B., Zeeck, A., Sheldrick, G.M.(2008) Acta Crystallogr D Biol Crystallogr 64: 985-992
- PubMed: 18703848
- DOI: https://doi.org/10.1107/S0907444908022646
- Primary Citation of Related Structures:
2V9B, 3C8P - PubMed Abstract:
Crystals of the cytotoxic thionin proteins viscotoxins A1 and B2 extracted from mistletoe diffracted to high resolution (1.25 and 1.05 A, respectively) and are excellent candidates for testing crystallographic methods. Ab initio direct methods were only successful in solving the viscotoxin B2 structure, which with 861 unique non-H atoms is one of the largest unknown structures without an atom heavier than sulfur to be solved in this way, but sulfur-SAD phasing provided a convincing solution for viscotoxin A1. Both proteins form dimers in the crystal and viscotoxin B2 (net charge +4 per monomer), but not viscotoxin A1 (net charge +6), is coordinated by sulfate or phosphate anions. The viscotoxin A1 crystal has a higher solvent content than the viscotoxin B2 crystal (49% as opposed to 28%) with solvent channels along the crystallographic 4(3) axes.
Organizational Affiliation:
Lehrstuhl für Strukturchemie, Georg-August-Universität, Tammannstrasse 4, D-37077 Göttingen, Germany.