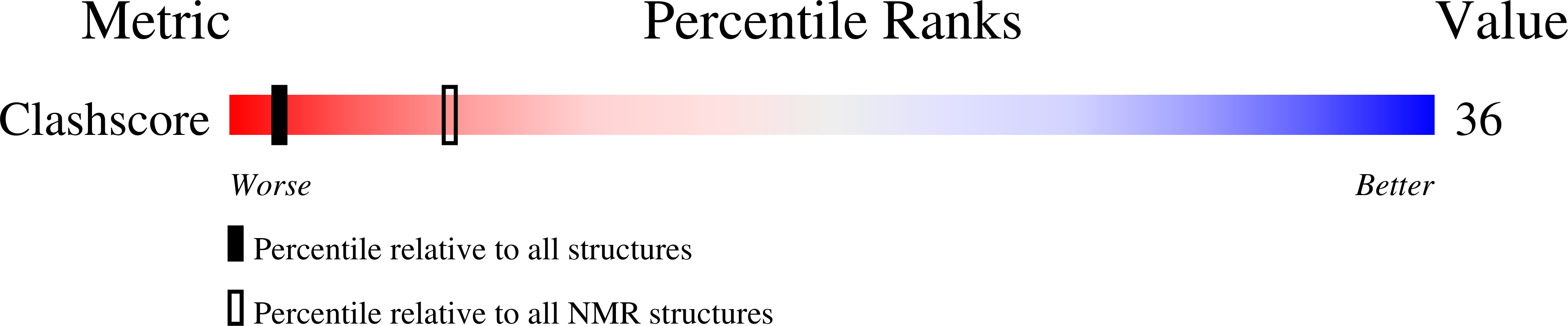Orientational and Dynamical Heterogeneity of Rhodamine 6G Terminally Attached to a DNA Helix Revealed by NMR and Single-Molecule Fluorescence Spectroscopy.
Neubauer, H., Gaiko, N., Berger, S., Schaffer, J., Eggeling, C., Tuma, J., Verdier, L., Seidel, C.A., Griesinger, C., Volkmer, A.(2007) J Am Chem Soc 129: 12746
- PubMed: 17900110
- DOI: https://doi.org/10.1021/ja0722574
- Primary Citation of Related Structures:
2V3L - PubMed Abstract:
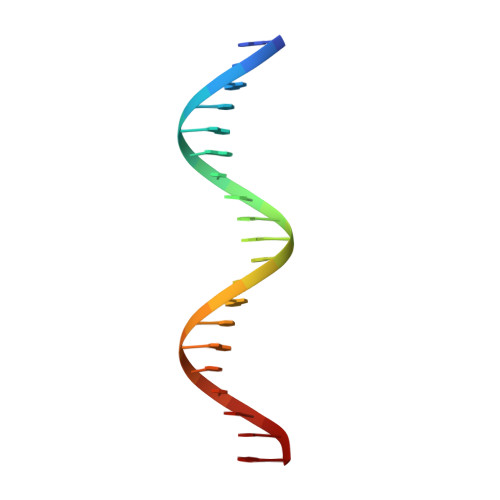
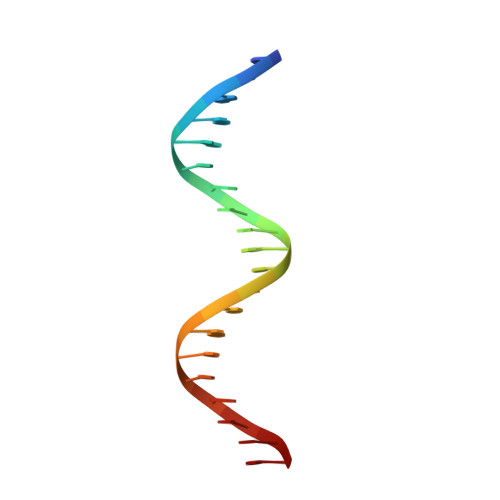
The comparison of Förster resonance energy transfer (FRET) efficiencies between two fluorophores covalently attached to a single protein or DNA molecule is an elegant approach for deducing information about their structural and dynamical heterogeneity. For a more detailed structural interpretation of single-molecule FRET assays, information about the positions as well as the dynamics of the dye labels attached to the biomolecule is important. In this work, Rhodamine 6G (2-[3'-(ethylamino)-6'-(ethylimino)-2',7'-dimethyl-6'H-xanthen-9'-yl]-benzoic acid) bound to the 5'-end of a 20 base pair long DNA duplex is investigated by both single-molecule multiparameter fluorescence detection (MFD) experiments and NMR spectroscopy. Rhodamine 6G is commonly employed in nucleic acid research as a FRET dye. MFD experiments directly reveal the equilibrium of the dye bound to DNA between three heterogeneous environments, which are characterized by distinct fluorescence lifetime and intensity distributions as a result of different guanine-dye excited-state electron transfer interactions. Sub-ensemble fluorescence autocorrelation analysis shows the highly dynamic character of the dye-DNA interactions ranging from nano- to milliseconds and species-specific triplet relaxation times. Two-dimensional NMR spectroscopy corroborates this information by the determination of the detailed geometric structures of the dye-nucleobase complex and their assignment to each population observed in the single-molecule fluorescence experiments. From both methods, a consistent and detailed molecular description of the structural and dynamical heterogeneity is obtained.
Organizational Affiliation:
Max-Planck-Institut für Biophysikalische Chemie, Am Fassberg 11, 37077, Göttingen, Germany.