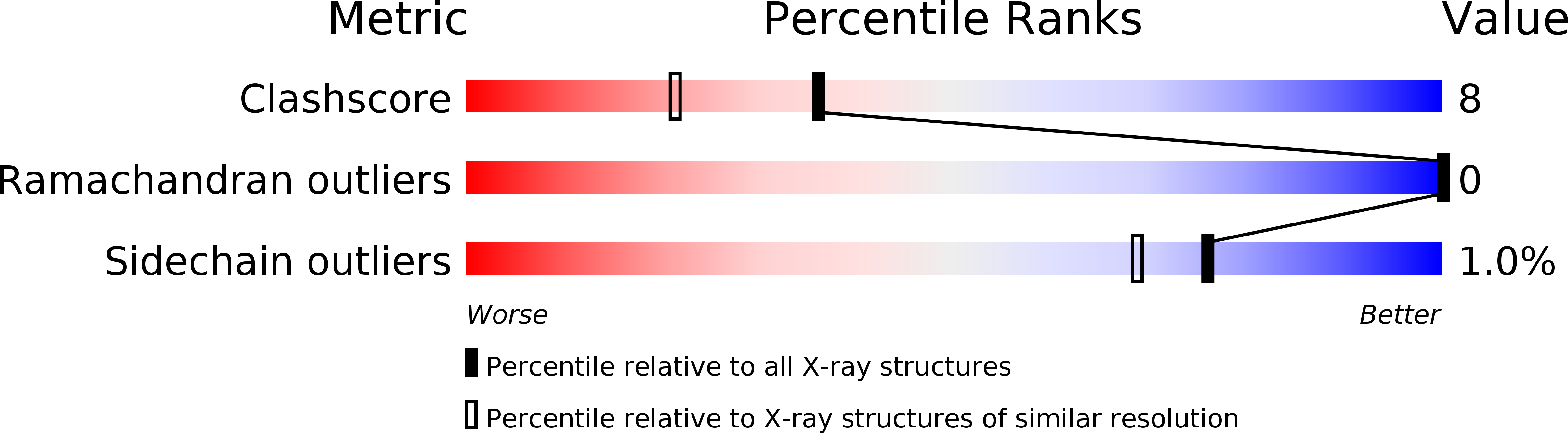Thermodynamic and structural consequences of changing a sulfur atom to a methylene group in the M13Nle mutation in ribonuclease-S.
Thomson, J., Ratnaparkhi, G.S., Varadarajan, R., Sturtevant, J.M., Richards, F.M.(1994) Biochemistry 33: 8587-8593
- PubMed: 8031793
- DOI: https://doi.org/10.1021/bi00194a025
- Primary Citation of Related Structures:
2RLN - PubMed Abstract:
Two fragments of pancreatic ribonuclease A, a truncated version of S-peptide (residues 1-15) and S-protein (residues 21-124), combine to give a catalytically active complex. We have substituted the wild-type residue at position 13, methionine (Met), with norleucine (Nle), where the only covalent change is the replacement of the sulfur atom with a methylene group. The thermodynamic parameters associated with the binding of this variant to S-protein, determined by titration calorimetry in the temperature range 10-40 degrees C, are reported and compared to values previously reported [Varadarajan, R., Connelly, P. R., Sturtevant, J. M., & Richards, F. M. (1992) Biochemistry 31, 1421-1426] for other position 13 analogs. The differences in the free energy and enthalpy of binding between the Met and Nle peptides are 0.6 and 7.9 kcal/mol at 25 degrees C, respectively. These differences are slightly larger than, but comparable to, the differences in the values for the Met/Ile and Met/Leu pairs. The structure of the mutant complex was determined to 1.85 A resolution and refined to an R-factor of 17.4%. The structures of mutant and wild-type complexes are practically identical although the Nle side chain has a significantly higher average B-factor than the corresponding Met side chain. In contrast, the B-factors of the atoms of the cage of residues surrounding position 13 are all somewhat lower in the Nle variant than the Met wild-type.(ABSTRACT TRUNCATED AT 250 WORDS)
Organizational Affiliation:
Department of Chemistry, Yale University, New Haven, Connecticut 06511.