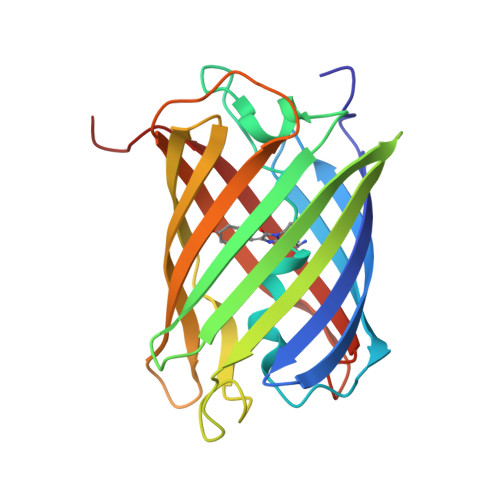
A structural basis for the pH-dependent increase in fluorescence efficiency of chromoproteins
Battad, J.M., Wilmann, P.G., Olsen, S., Byres, E., Smith, S.C., Dove, S.G., Turcic, K.N., Devenish, R.J., Rossjohn, J., Prescott, M.(2007) J Mol Biol 368: 998-1010
- PubMed: 17376484
- DOI: https://doi.org/10.1016/j.jmb.2007.02.007
- Primary Citation of Related Structures:
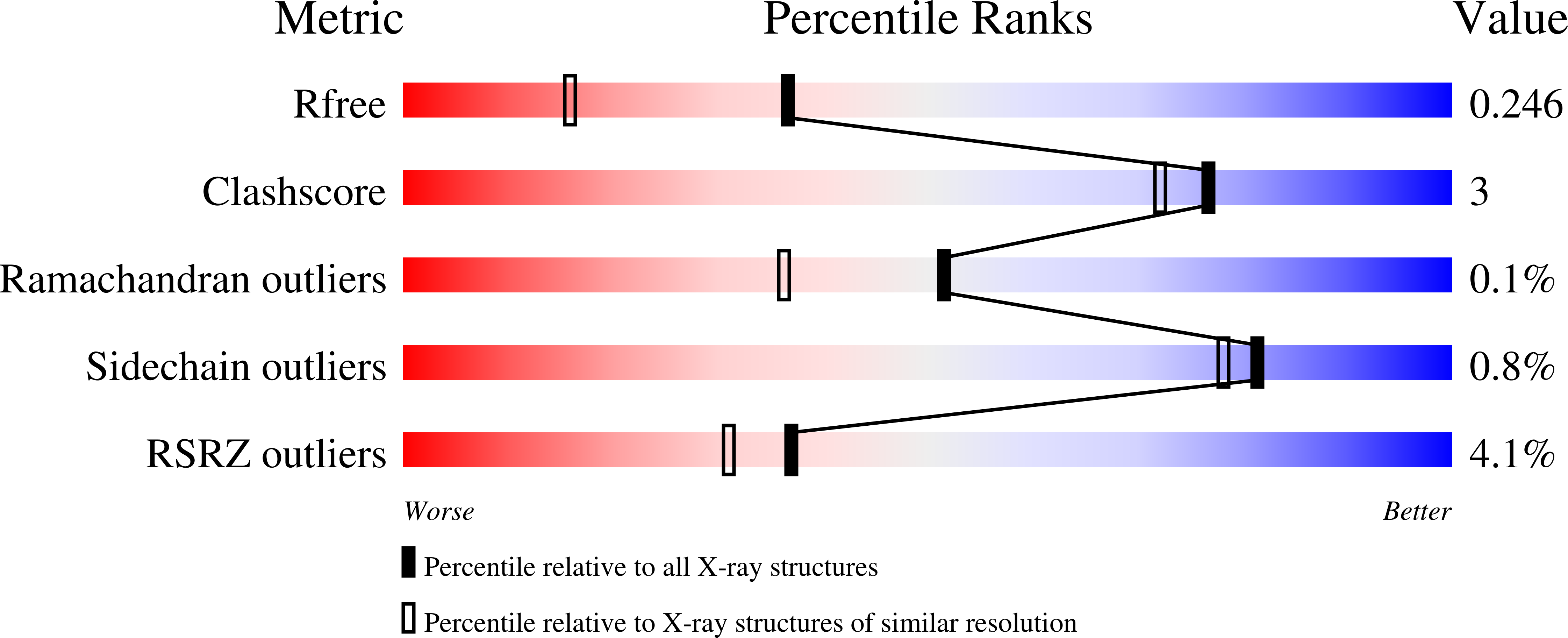
2P4M - PubMed Abstract:
Within the fluorescent protein and chromoprotein family, the phenomenon of photoswitching is both intriguing and biotechnologically useful. Illumination of particular chromoproteins with intense light results in dramatic increases in fluorescence efficiency (termed kindling) and involves cis-trans isomerization of the chromophore. Here we report that chromophore isomerization can also be driven via alteration in pH. Specifically, we demonstrate that a number of naturally occurring chromoproteins, and their engineered variants, undergo a dramatic 20-100-fold increase in fluorescence efficiency at alkaline pH (>pH9.0). We have determined to 1.8 A resolution the structure of one such chromoprotein, Rtms5(H146S), in its highly far-red fluorescent form (Phi(F), 0.11 at pH 10.7) and compared it to the structure of the non-fluorescent form (Phi(F), 0.002 at pH 8.0). At high pH, the cyclic tri-peptide chromophore was observed to be mobile and distributed between a trans non-coplanar and a cis coplanar conformation, whereas at the lower pH, only a trans non-coplanar chromophore was observed. Calculation of pK(a) values suggested that titration of the side-chain of the conserved Glu215 close to the chromophore is involved in promoting the cis-coplanar conformation. Collectively, our data establish that isomerization to form a coplanar chromophore is a basis of the increased fluorescence efficiency at high pH. The phenomenon of pH-induced fluorescence gain has similarities with photoswitching, thereby providing a model to study the mechanism of kindling.
Organizational Affiliation:
Department of Biochemistry and Molecular Biology, School of Biomedical Sciences, Monash University, Clayton Campus, Victoria 3800, Australia.