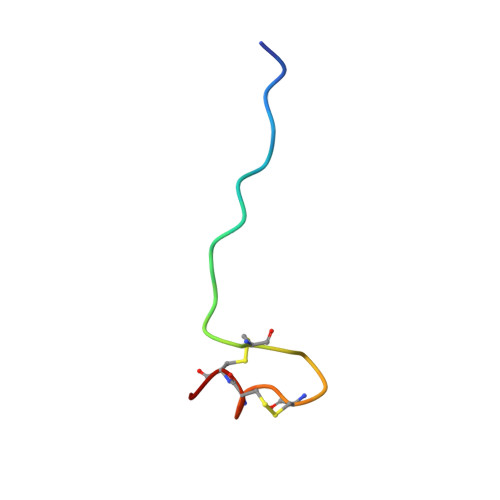
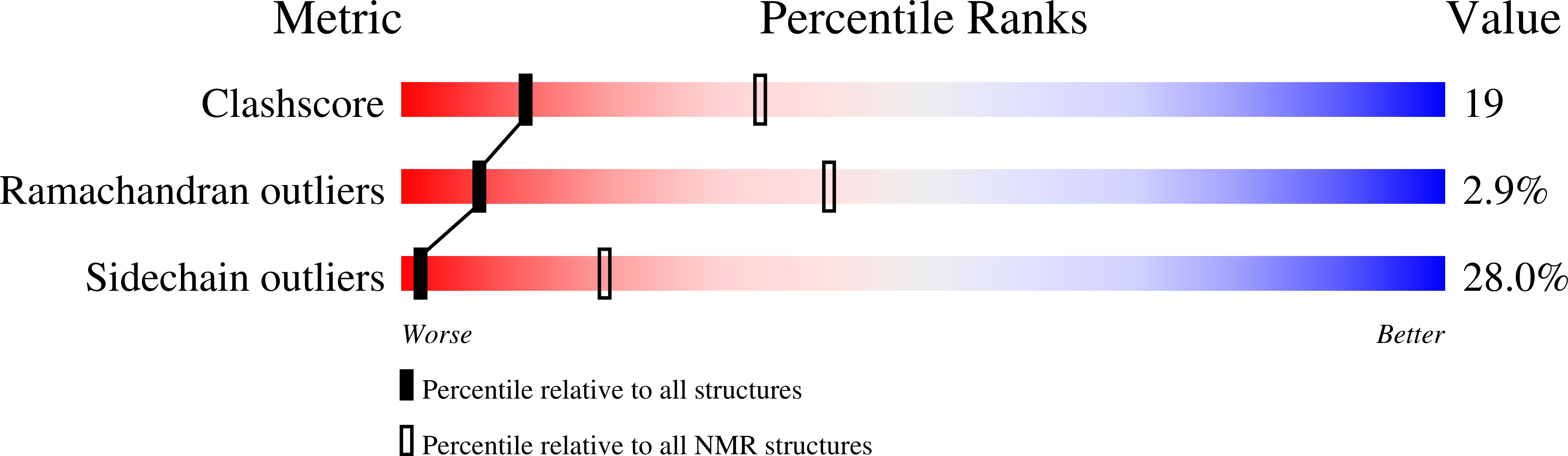Minicollagen-15, a novel minicollagen isolated from Hydra, forms tubule structures in nematocysts.
Adamczyk, P., Meier, S., Gross, T., Hobmayer, B., Grzesiek, S., Bachinger, H.P., Holstein, T.W., Ozbek, S.(2008) J Mol Biol 376: 1008-1020
- PubMed: 18206162
- DOI: https://doi.org/10.1016/j.jmb.2007.10.090
- Primary Citation of Related Structures:
2OQ9 - PubMed Abstract:
Minicollagens constitute a family of unusually short collagen molecules isolated from cnidarians. They are restricted to the nematocyst, a cylindrical explosive organelle serving in defense and capture of prey. The nematocyst capsule contains a long tubule inside of its matrix, which is expelled and everted during an ultrafast discharge process. Here, we report the cloning and characterization of a novel minicollagen in Hydra, designated minicollagen-15 (NCol-15). NCol-15, like NCol-3 and NCol-4, shows deviations from the canonical cysteine pattern in its terminal cysteine-rich domains (CRDs). Minicollagens share common domain architectures with a central collagen sequence flanked by polyproline stretches and short N- and C-terminal CRDs. The CRDs are involved in the formation of a highly resistant cysteine network, which constitutes the basic structure of the nematocyst capsule. Unlike NCol-1, which is part of the capsule wall, NCol-15 is localized to tubules, arguing for a functional differentiation of minicollagens within the nematocyst architecture. NMR analysis of the altered C-terminal CRD of NCol-15 showed a novel disulfide-linked structure within the cysteine-containing region exhibiting similar folding kinetics and stability as the canonical CRDs. Our data provide evidence for evolutionary diversification among minicollagens, which probably facilitated alterations in the morphology of the nematocyst wall and tubule.
Organizational Affiliation:
Institute of Zoology, Department of Molecular Evolution and Genomics, Im Neuenheimer Feld 230, 69130 Heidelberg, Germany.