Structure of amylase-binding protein A of Streptococcus gordonii: A potential receptor for human salivary alpha-amylase enzyme.
Sethi, A., Mohanty, B., Ramasubbu, N., Gooley, P.R.(2015) Protein Sci 24: 1013-1018
- PubMed: 25739638
- DOI: https://doi.org/10.1002/pro.2671
- Primary Citation of Related Structures:
2MXX - PubMed Abstract:
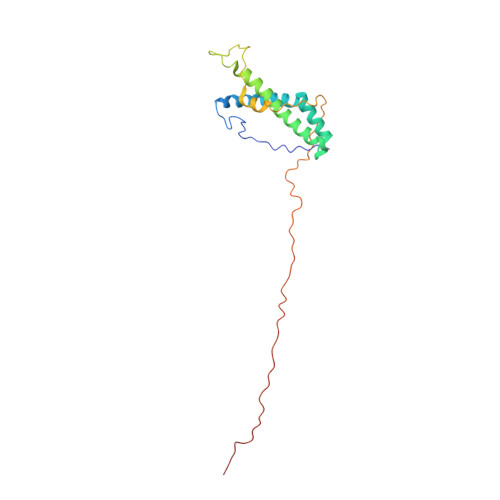
Amylase-binding protein A (AbpA) of a number of oral streptococci is essential for the colonization of the dental pellicle. We have determined the solution structure of residues 24-195 of AbpA of Streptococcus gordonii and show a well-defined core of five helices in the region of 45-115 and 135-145. (13) Cα/β chemical shift and heteronuclear (15) N-{(1) H} NOE data are consistent with this fold and that the remainder of the protein is unstructured. The structure will inform future molecular experiments in defining the mechanism of human salivary α-amylase binding and biofilm formation by streptococci.
Organizational Affiliation:
Department of Biochemistry and Molecular Biology, Bio21 Molecular Science and Biotechnology Institute, University of Melbourne, 30 Flemington Road, Parkville, Victoria, 3010, Australia.