Homing endonuclease I-CreI derivatives with novel DNA target specificities.
Rosen, L.E., Morrison, H.A., Masri, S., Brown, M.J., Springstubb, B., Sussman, D., Stoddard, B.L., Seligman, L.M.(2006) Nucleic Acids Res 34: 4791-4800
- PubMed: 16971456
- DOI: https://doi.org/10.1093/nar/gkl645
- Primary Citation of Related Structures:
2I3P, 2I3Q - PubMed Abstract:
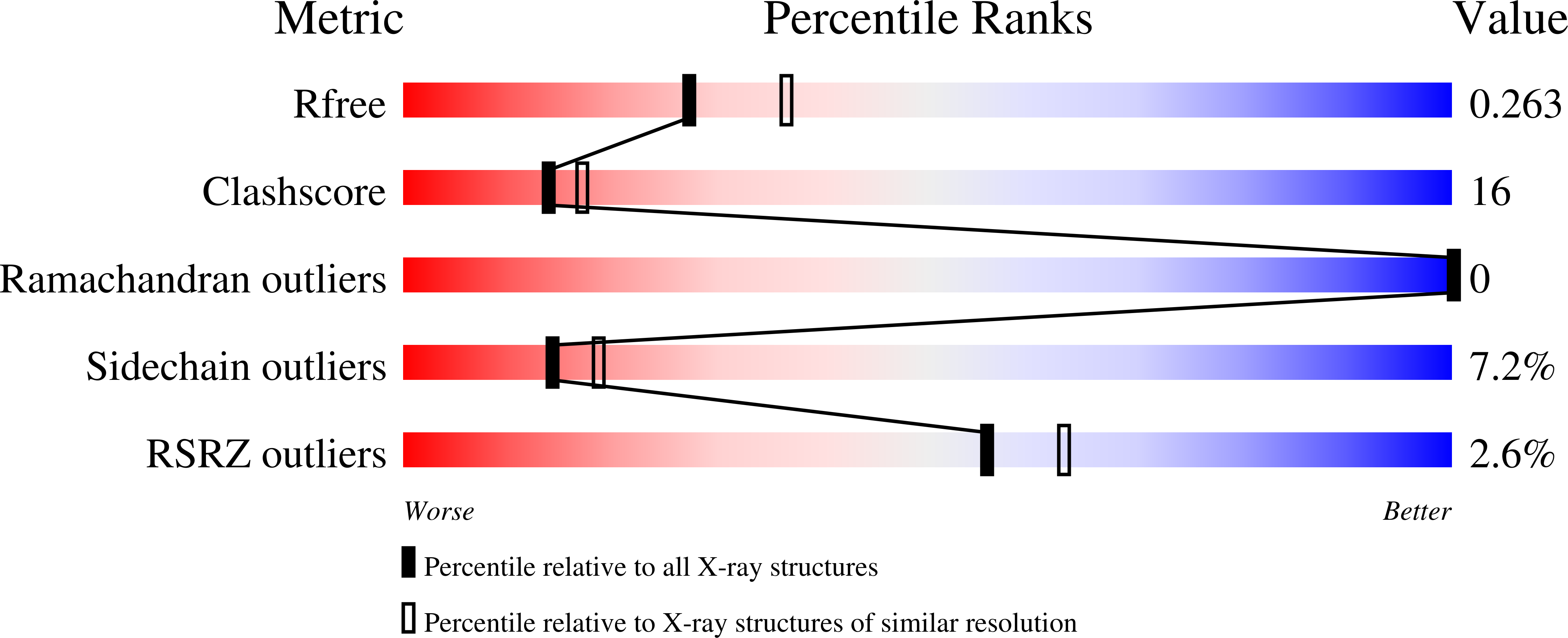
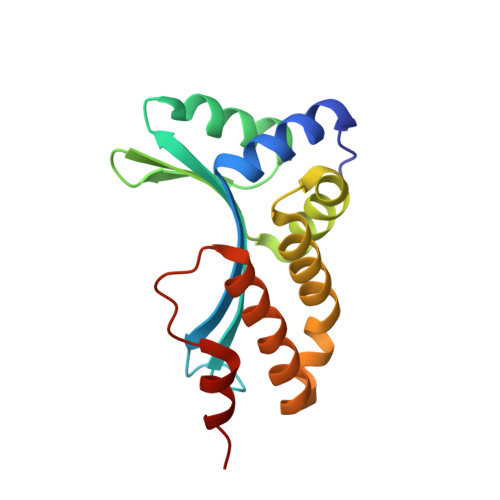
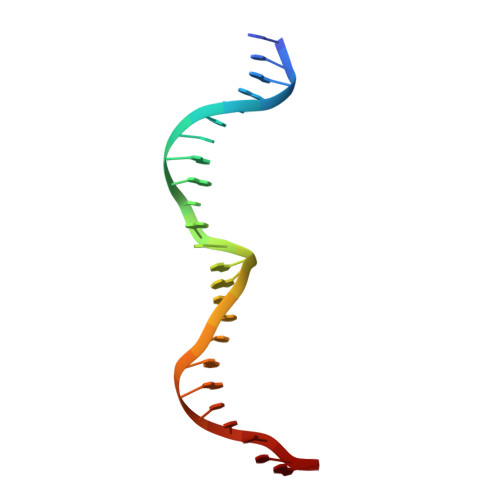
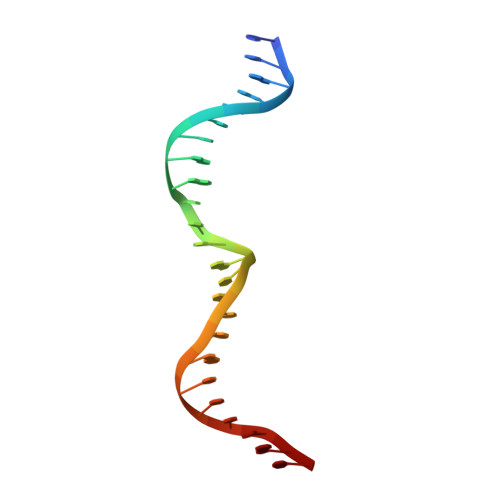
Homing endonucleases are highly specific enzymes, capable of recognizing and cleaving unique DNA sequences in complex genomes. Since such DNA cleavage events can result in targeted allele-inactivation and/or allele-replacement in vivo, the ability to engineer homing endonucleases matched to specific DNA sequences of interest would enable powerful and precise genome manipulations. We have taken a step-wise genetic approach in analyzing individual homing endonuclease I-CreI protein/DNA contacts, and describe here novel interactions at four distinct target site positions. Crystal structures of two mutant endonucleases reveal the molecular interactions responsible for their altered DNA target specificities. We also combine novel contacts to create an endonuclease with the predicted target specificity. These studies provide important insights into engineering homing endonucleases with novel target specificities, as well as into the evolution of DNA recognition by this fascinating family of proteins.
Organizational Affiliation:
Department of Biology and Program in Molecular Biology, Pomona College, 175 West 6th Street, Claremont, CA 91711, USA.