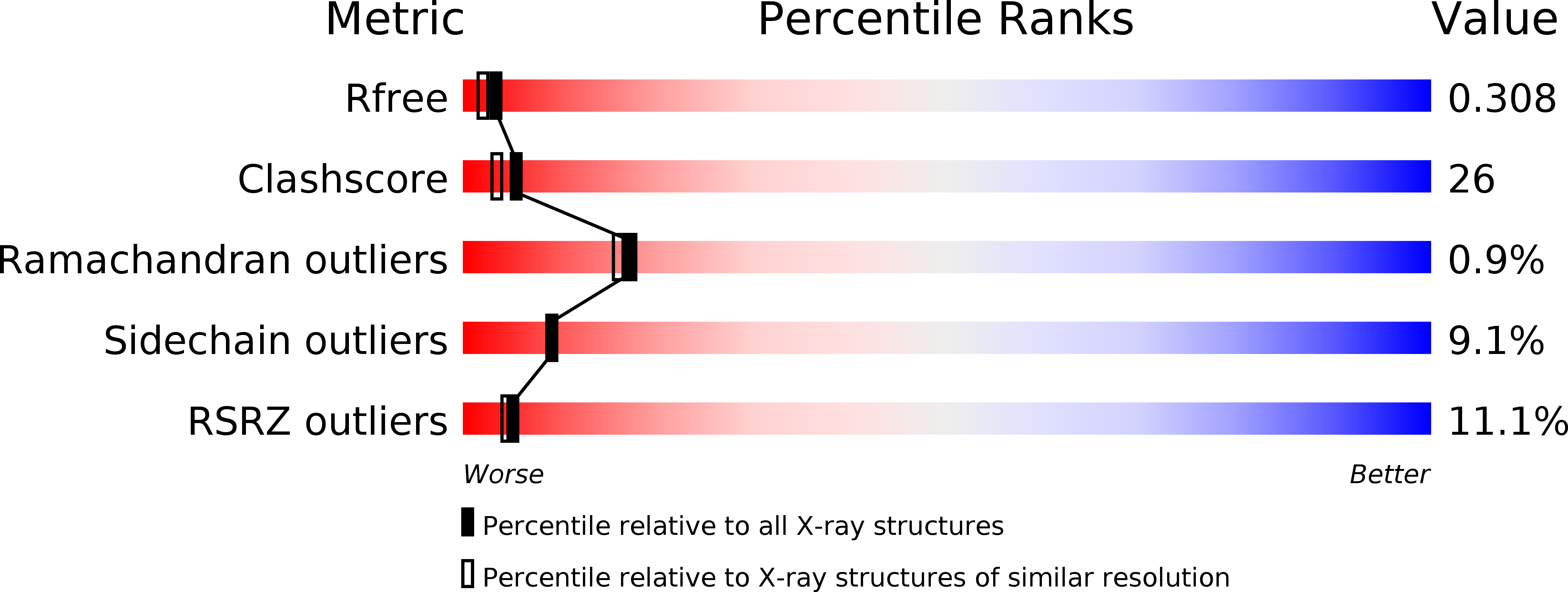
The discovery of tetrahydrofluorenones as a new class of estrogen receptor beta-subtype selective ligands.
Wilkening, R.R., Ratcliffe, R.W., Tynebor, E.C., Wildonger, K.J., Fried, A.K., Hammond, M.L., Mosley, R.T., Fitzgerald, P.M.D., Sharma, N., McKeever, B.M., Nilsson, S., Carlquist, M., Thorsell, A., Locco, L., Katz, R., Frisch, K., Birzin, E.T., Wilkinson, H.A., Mitra, S., Cai, S., Hayes, E.C., Schaeffer, J.M., Rohrer, S.P.(2006) Bioorg Med Chem Lett 16: 3489-3494
- PubMed: 16632357
- DOI: https://doi.org/10.1016/j.bmcl.2006.03.098
- Primary Citation of Related Structures:
2GIU - PubMed Abstract:
Synthesis and derivatization of a series of substituted tetrahydrofluorenone analogs giving potent, ERbeta subtype selective ligands are described. Several analogs possessing ERbeta binding affinities comparable to 17beta-estradiol but with greater than 75-fold selectivity over ERalpha are reported.
Organizational Affiliation:
Department of Medicinal Chemistry, Merck Research Laboratories, PO Box 2000, Rahway, NJ 07065, USA. robert_wilkening@merck.com