Potential Regulatory Interactions of Escherichia Coli Rraa Protein with Dead-Box Helicases.
Pietras, Z., Hardwick, S.W., Swiezewski, S., Luisi, B.F.(2013) J Biol Chem 288: 31919
- PubMed: 24045937
- DOI: https://doi.org/10.1074/jbc.M113.502146
- Primary Citation of Related Structures:
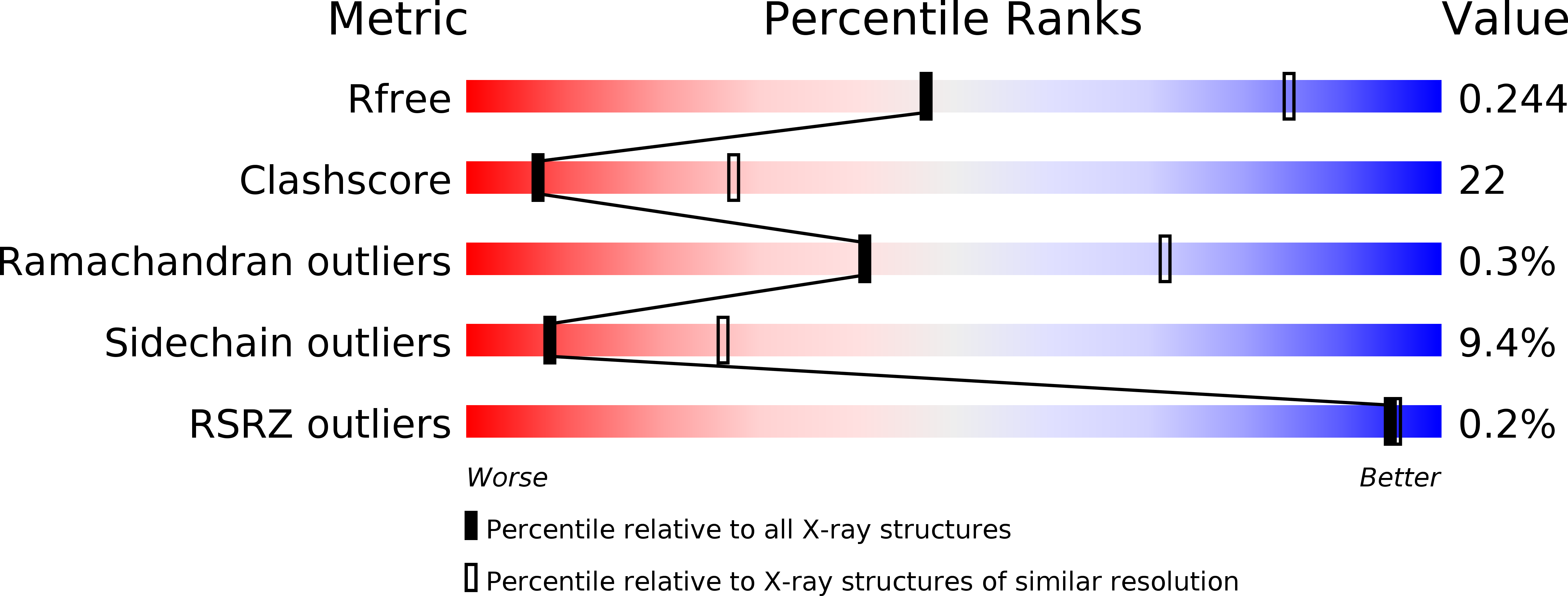
2YJT, 2YJV - PubMed Abstract:
Members of the DEAD-box family of RNA helicases contribute to virtually every aspect of RNA metabolism, in organisms from all domains of life. Many of these helicases are constituents of multicomponent assemblies, and their interactions with partner proteins within the complexes underpin their activities and biological function. In Escherichia coli the DEAD-box helicase RhlB is a component of the multienzyme RNA degradosome assembly, and its interaction with the core ribonuclease RNase E boosts the ATP-dependent activity of the helicase. Earlier studies have identified the regulator of ribonuclease activity A (RraA) as a potential interaction partner of both RNase E and RhlB. We present structural and biochemical evidence showing how RraA can bind to, and modulate the activity of RhlB and another E. coli DEAD-box enzyme, SrmB. Crystallographic structures are presented of RraA in complex with a portion of the natively unstructured C-terminal tail of RhlB at 2.8-Å resolution, and in complex with the C-terminal RecA-like domain of SrmB at 2.9 Å. The models suggest two distinct mechanisms by which RraA might modulate the activity of these and potentially other helicases.
Organizational Affiliation:
From the Department of Biochemistry, University of Cambridge, Tennis Court Road, Cambridge CB2 1GA, United Kingdom and.