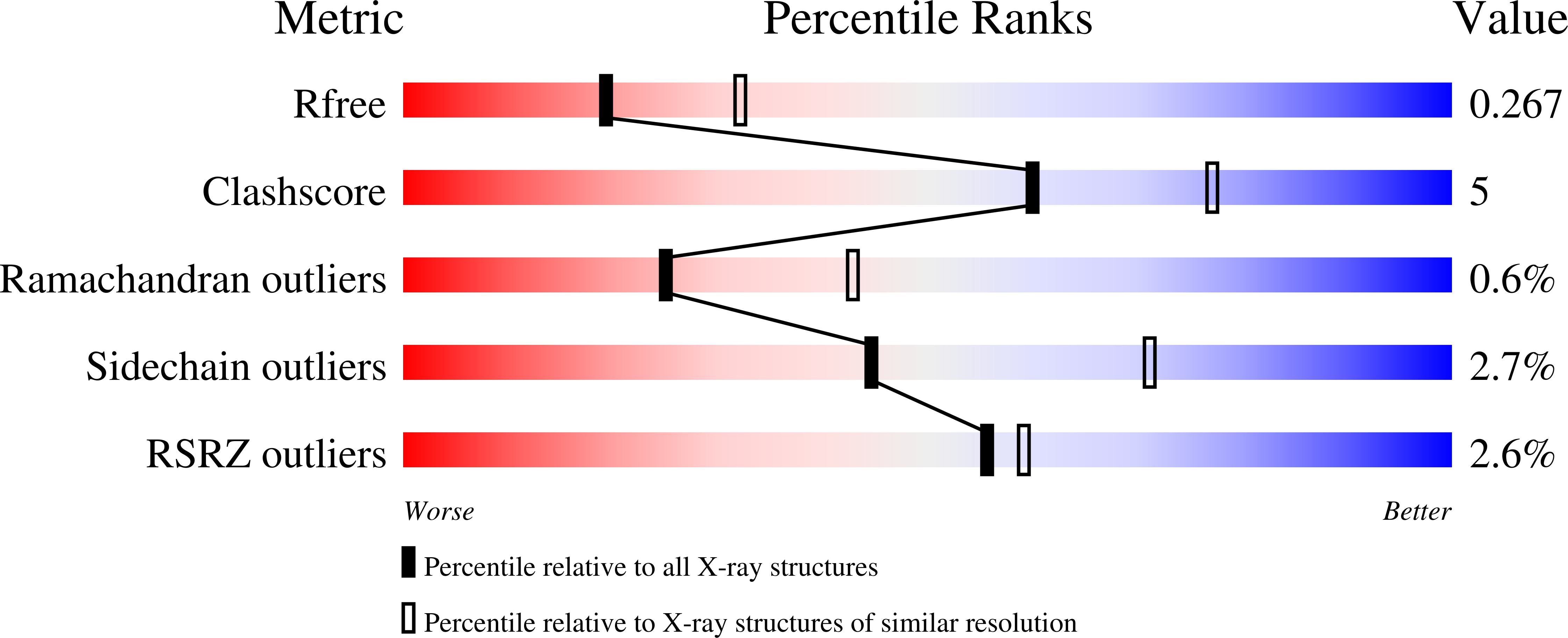
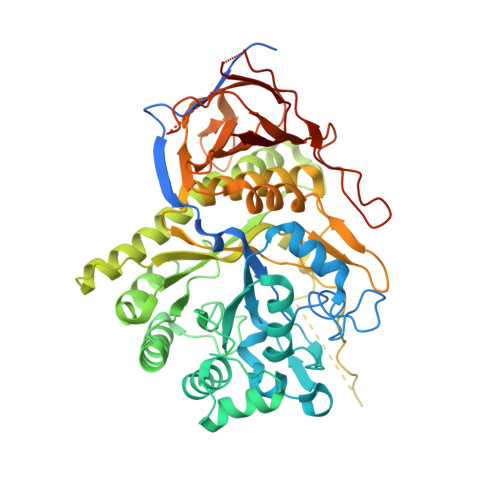
Elucidation of the Substrate Specificity and Protein Structure of Abfb, a Family 51 Alpha-L- Arabinofuranosidase from Bifidobacterium Longum.
Lagaert, S., Schoepe, J., Delcour, J.A., Lavigne, R., Strelkov, S.V., Courtin, C.M., Mikkelsen, N.E., Sandgren, M., Volckaert, G.To be published.