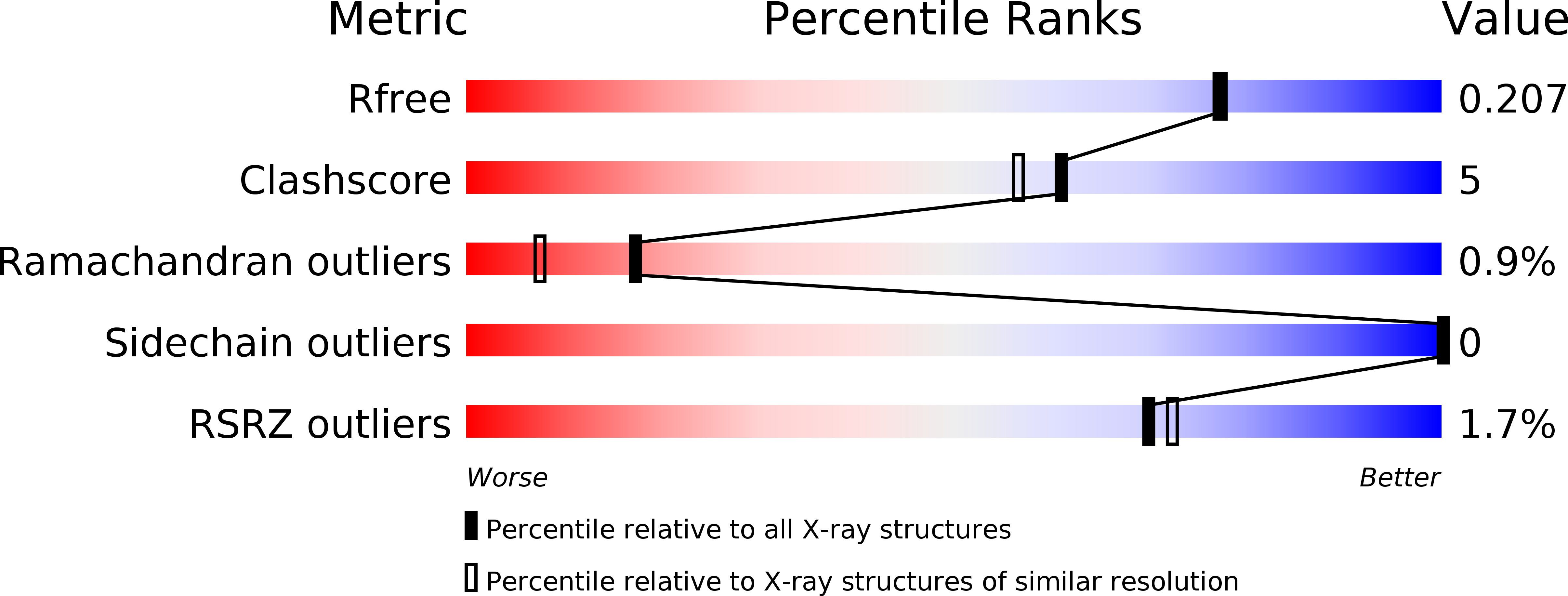
Burkholderia Cenocepacia Bc2L-C is a Super Lectin with Dual Specificity and Proinflammatory Activity.
Sulak, O., Cioci, G., Lameignere, E., Balloy, V., Round, A., Gutsche, I., Malinovska, L., Chignard, M., Kosma, P., Aubert, D.F., Marolda, C.L., Valvano, M.A., Wimmerova, M., Imberty, A.(2011) PLoS Pathog 7: 2238
- PubMed: 21909279
- DOI: https://doi.org/10.1371/journal.ppat.1002238
- Primary Citation of Related Structures:
2XR4 - PubMed Abstract:
Lectins and adhesins are involved in bacterial adhesion to host tissues and mucus during early steps of infection. We report the characterization of BC2L-C, a soluble lectin from the opportunistic pathogen Burkholderia cenocepacia, which has two distinct domains with unique specificities and biological activities. The N-terminal domain is a novel TNF-α-like fucose-binding lectin, while the C-terminal part is similar to a superfamily of calcium-dependent bacterial lectins. The C-terminal domain displays specificity for mannose and l-glycero-d-manno-heptose. BC2L-C is therefore a superlectin that binds independently to mannose/heptose glycoconjugates and fucosylated human histo-blood group epitopes. The apo form of the C-terminal domain crystallized as a dimer, and calcium and mannose could be docked in the binding site. The whole lectin is hexameric and the overall structure, determined by electron microscopy and small angle X-ray scattering, reveals a flexible arrangement of three mannose/heptose-specific dimers flanked by two fucose-specific TNF-α-like trimers. We propose that BC2L-C binds to the bacterial surface in a mannose/heptose-dependent manner via the C-terminal domain. The TNF-α-like domain triggers IL-8 production in cultured airway epithelial cells in a carbohydrate-independent manner, and is therefore proposed to play a role in the dysregulated proinflammatory response observed in B. cenocepacia lung infections. The unique architecture of this newly recognized superlectin correlates with multiple functions including bacterial cell cross-linking, adhesion to human epithelia, and stimulation of inflammation.
Organizational Affiliation:
CERMAV-CNRS- UPR5301 affiliated to Université Joseph Fourier, Grenoble, France.