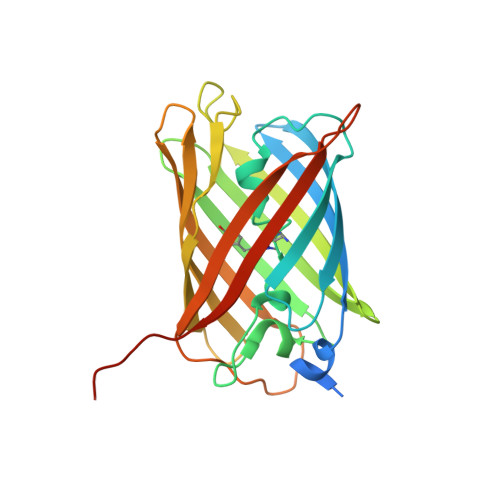
Structural Basis for the Phototoxicity of the Fluorescent Protein Killerred.
Carpentier, P., Violot, S., Blanchoin, L., Bourgeois, D.(2009) FEBS Lett 583: 2839
- PubMed: 19646983
- DOI: https://doi.org/10.1016/j.febslet.2009.07.041
- Primary Citation of Related Structures:
2WIQ, 2WIS - PubMed Abstract:
The red fluorescent protein KillerRed, engineered from the hydrozoan chromoprotein anm2CP, has been reported to induce strong cytotoxicity through the chromophore assisted light inactivation (CALI) effect. Here, we present the X-ray structures of KillerRed in its native and bleached states. A long water-filled channel is revealed, connecting the methylene bridge of the chromophore to the solvent. This channel facilitates the transit of oxygen and of reactive oxygen species (ROS) formed by reaction with the excited chromophore. The functional roles of key mutations used to produce KillerRed are discussed, strong chromophore distortions in the bleached state are revealed, and mechanisms for ROS production and self protection are proposed. The presence of a partially mature, photo-resistant, green-emitting state is characterized, which accounts for enhanced CALI by "pre-bleached" KillerRed.
Organizational Affiliation:
Laboratoire de Cristallographie et Cristallogenèse des Protéines, IBS, Institut de Biologie Structurale Jean-Pierre Ebel, CEA, CNRS, Université Joseph Fourier, Grenoble, France. philippe.carpentier@ibs.fr