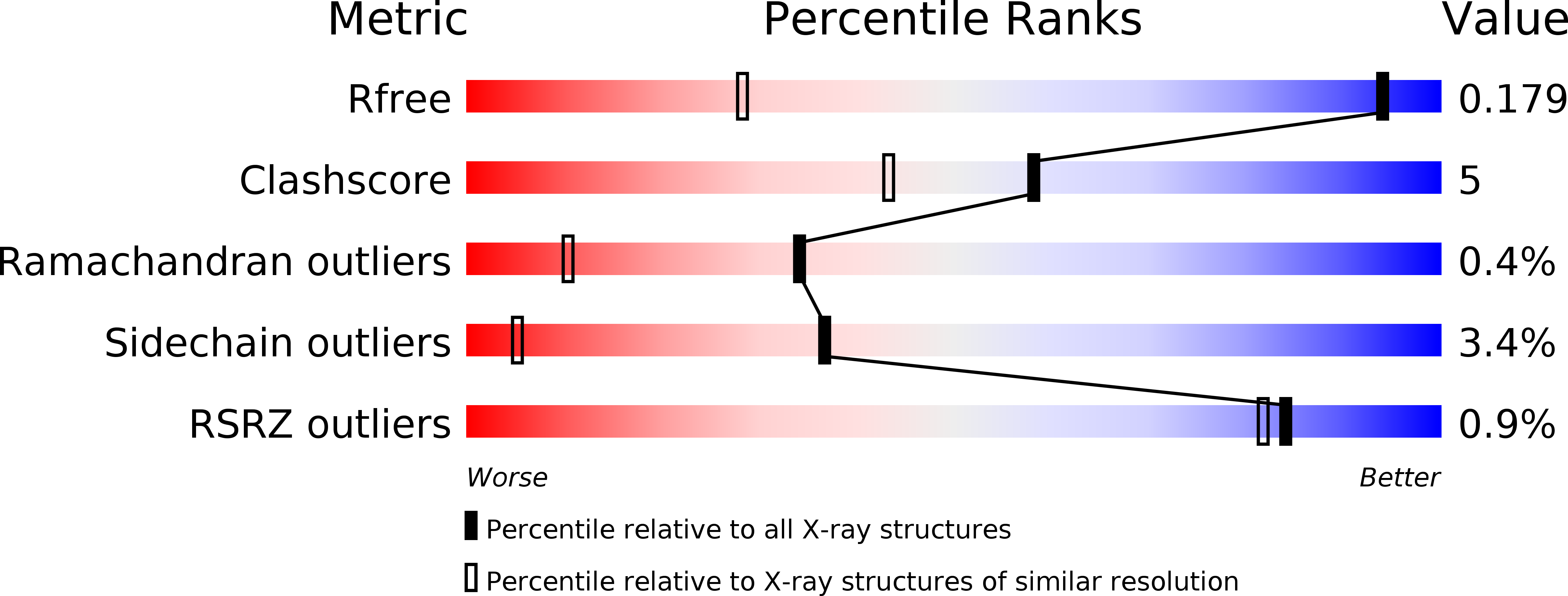
Structure of GES-1 at atomic resolution: insights into the evolution of carbapenamase activity in the class A extended-spectrum beta-lactamases.
Smith, C.A., Caccamo, M., Kantardjieff, K.A., Vakulenko, S.(2007) Acta Crystallogr D Biol Crystallogr 63: 982-992
- PubMed: 17704567
- DOI: https://doi.org/10.1107/S0907444907036955
- Primary Citation of Related Structures:
2QPN - PubMed Abstract:
The structure of the class A extended-spectrum beta-lactamase GES-1 from Klebsiella pneumoniae has been determined to 1.1 A resolution. GES-1 has the characteristic active-site disulfide bond of the carbapenemase family of beta-lactamases and has a structure that is very similar to those of other known carbapenemases, including NMC-A, SME-1 and KPC-2. Most residues implicated in the catalytic mechanism of this class of enzyme are present in the GES-1 active site, including Ser70, which forms a covalent bond with the carbonyl C atom of the beta-lactam ring of the substrate during the formation of an acyl-enzyme intermediate, Glu166, which is implicated as both the acylation and deacylation base, and Lys73, which is also implicated as the acylation base. A water molecule crucial to catalysis is observed in an identical location as in other class A beta-lactamases, interacting with the side chains of Ser70 and Glu166. One important residue, Asn170, also normally a ligand for the hydrolytic water, is missing from the GES-1 active site. This residue is a glycine in GES-1 and the enzyme is unable to hydrolyze imipenem. This points to this residue as being critically important in the hydrolysis of this class of beta-lactam substrate. This is further supported by flexible-docking studies of imipenem with in silico-generated Gly170Asn and Gly170Ser mutant GES-1 enzymes designed to mimic the active sites of imipenem-hydrolyzing point mutants GES-2 and GES-5.
Organizational Affiliation:
Stanford Synchrotron Radiation Laboratory, Stanford University, Menlo Park, CA, USA. csmith@slac.stanford.edu