Structural insights into the interaction of the evolutionarily conserved ZPR1 domain tandem with eukaryotic EF1A, receptors, and SMN complexes.
Mishra, A.K., Gangwani, L., Davis, R.J., Lambright, D.G.(2007) Proc Natl Acad Sci U S A 104: 13930-13935
- PubMed: 17704259
- DOI: https://doi.org/10.1073/pnas.0704915104
- Primary Citation of Related Structures:
2QKD - PubMed Abstract:
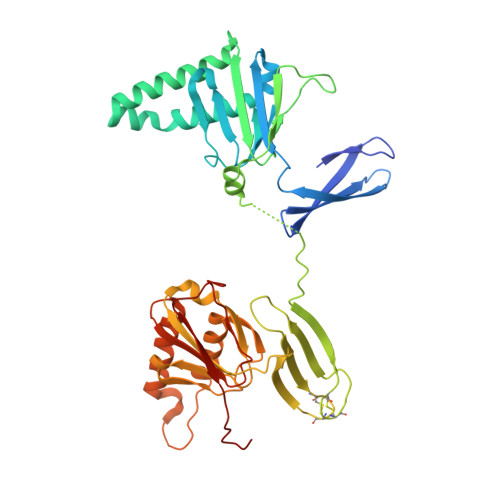
Eukaryotic genomes encode a zinc finger protein (ZPR1) with tandem ZPR1 domains. In response to growth stimuli, ZPR1 assembles into complexes with eukaryotic translation elongation factor 1A (eEF1A) and the survival motor neurons protein. To gain insight into the structural mechanisms underlying the essential function of ZPR1 in diverse organisms, we determined the crystal structure of a ZPR1 domain tandem and characterized the interaction with eEF1A. The ZPR1 domain consists of an elongation initiation factor 2-like zinc finger and a double-stranded beta helix with a helical hairpin insertion. ZPR1 binds preferentially to GDP-bound eEF1A but does not directly influence the kinetics of nucleotide exchange or GTP hydrolysis. However, ZPR1 efficiently displaces the exchange factor eEF1Balpha from preformed nucleotide-free complexes, suggesting that it may function as a negative regulator of eEF1A activation. Structure-based mutational and complementation analyses reveal a conserved binding epitope for eEF1A that is required for normal cell growth, proliferation, and cell cycle progression. Structural differences between the ZPR1 domains contribute to the observed functional divergence and provide evidence for distinct modalities of interaction with eEF1A and survival motor neuron complexes.
Organizational Affiliation:
Program in Molecular Medicine and Department of Biochemistry and Molecular Pharmacology, University of Massachusetts Medical School, Worcester, MA 01655, USA.