Novel Structural Components Contribute to the High Thermal Stability of Acyl Carrier Protein from Enterococcus faecalis.
Park, Y.G., Jung, M.C., Song, H., Jeong, K.W., Bang, E., Hwang, G.S., Kim, Y.(2016) J Biol Chem 291: 1692-1702
- PubMed: 26631734
- DOI: https://doi.org/10.1074/jbc.M115.674408
- Primary Citation of Related Structures:
2N50 - PubMed Abstract:
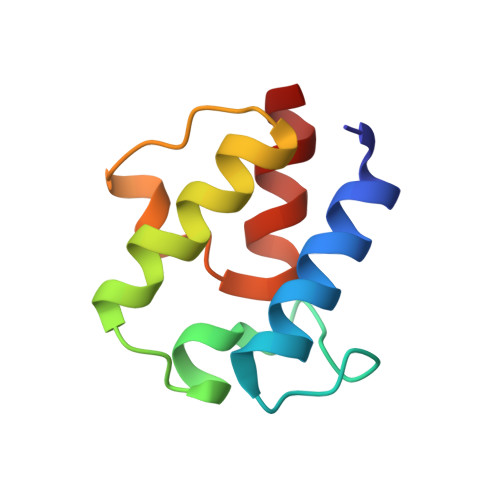
Enterococcus faecalis is a Gram-positive, commensal bacterium that lives in the gastrointestinal tracts of humans and other mammals. It causes severe infections because of high antibiotic resistance. E. faecalis can endure extremes of temperature and pH. Acyl carrier protein (ACP) is a key element in the biosynthesis of fatty acids responsible for acyl group shuttling and delivery. In this study, to understand the origin of high thermal stabilities of E. faecalis ACP (Ef-ACP), its solution structure was investigated for the first time. CD experiments showed that the melting temperature of Ef-ACP is 78.8 °C, which is much higher than that of Escherichia coli ACP (67.2 °C). The overall structure of Ef-ACP shows the common ACP folding pattern consisting of four α-helices (helix I (residues 3-17), helix II (residues 39-53), helix III (residues 60-64), and helix IV (residues 68-78)) connected by three loops. Unique Ef-ACP structural features include a hydrophobic interaction between Phe(45) in helix II and Phe(18) in the α1α2 loop and a hydrogen bonding between Ser(15) in helix I and Ile(20) in the α1α2 loop, resulting in its high thermal stability. Phe(45)-mediated hydrophobic packing may block acyl chain binding subpocket II entry. Furthermore, Ser(58) in the α2α3 loop in Ef-ACP, which usually constitutes a proline in other ACPs, exhibited slow conformational exchanges, resulting in the movement of the helix III outside the structure to accommodate a longer acyl chain in the acyl binding cavity. These results might provide insights into the development of antibiotics against pathogenic drug-resistant E. faecalis strains.
Organizational Affiliation:
From the Department of Bioscience and Biotechnology and the Bio/Molecular Informatics Center Konkuk University, Seoul 143-701, Korea and.