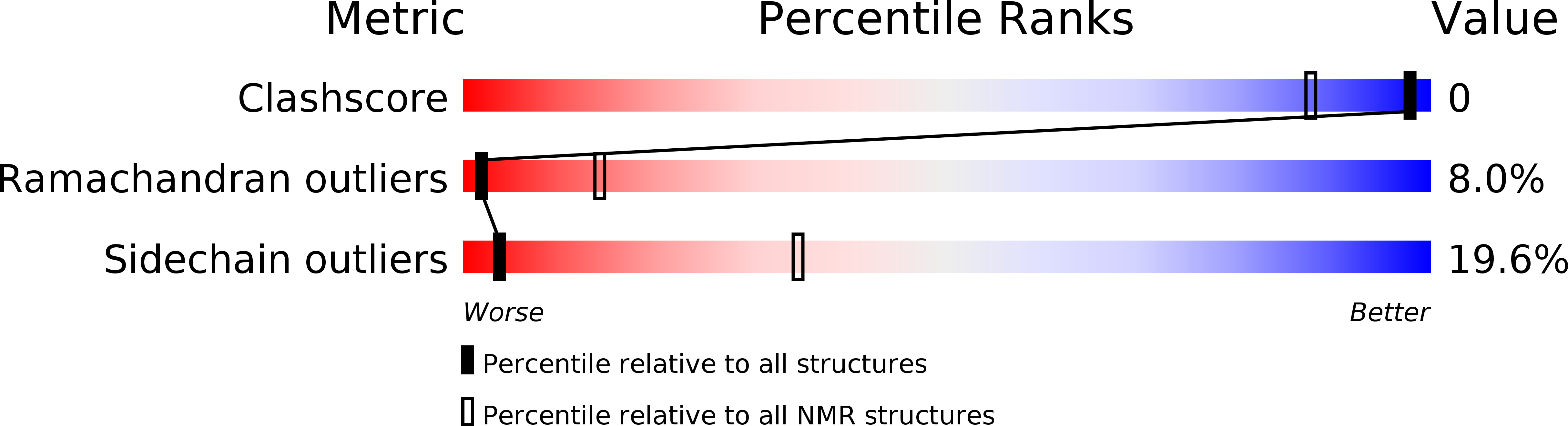Structural and functional diversity of acidic scorpion potassium channel toxins.
Chen, Z.Y., Zeng, D.Y., Hu, Y.T., He, Y.W., Pan, N., Ding, J.P., Cao, Z.J., Liu, M.L., Li, W.X., Yi, H., Jiang, L., Wu, Y.L.(2012) PLoS One 7: e35154-e35154
- PubMed: 22511981
- DOI: https://doi.org/10.1371/journal.pone.0035154
- Primary Citation of Related Structures:
2LIX - PubMed Abstract:
Although the basic scorpion K(+) channel toxins (KTxs) are well-known pharmacological tools and potential drug candidates, characterization the acidic KTxs still has the great significance for their potential selectivity towards different K(+) channel subtypes. Unfortunately, research on the acidic KTxs has been ignored for several years and progressed slowly. Here, we describe the identification of nine new acidic KTxs by cDNA cloning and bioinformatic analyses. Seven of these toxins belong to three new α-KTx subfamilies (α-KTx28, α-KTx29, and α-KTx30), and two are new members of the known κ-KTx2 subfamily. ImKTx104 containing three disulfide bridges, the first member of the α-KTx28 subfamily, has a low sequence homology with other known KTxs, and its NMR structure suggests ImKTx104 adopts a modified cystine-stabilized α-helix-loop-β-sheet (CS-α/β) fold motif that has no apparent α-helixs and β-sheets, but still stabilized by three disulfide bridges. These newly described acidic KTxs exhibit differential pharmacological effects on potassium channels. Acidic scorpion toxin ImKTx104 was the first peptide inhibitor found to affect KCNQ1 channel, which is insensitive to the basic KTxs and is strongly associated with human cardiac abnormalities. ImKTx104 selectively inhibited KCNQ1 channel with a K(d) of 11.69 µM, but was less effective against the basic KTxs-sensitive potassium channels. In addition to the ImKTx104 toxin, HeTx204 peptide, containing a cystine-stabilized α-helix-loop-helix (CS-α/α) fold scaffold motif, blocked both Kv1.3 and KCNQ1 channels. StKTx23 toxin, with a cystine-stabilized α-helix-loop-β-sheet (CS-α/β) fold motif, could inhibit Kv1.3 channel, but not the KCNQ1 channel. These findings characterize the structural and functional diversity of acidic KTxs, and could accelerate the development and clinical use of acidic KTxs as pharmacological tools and potential drugs.
Organizational Affiliation:
State Key Laboratory of Virology, College of Life Sciences, Wuhan University, Wuhan, People's Republic of China.