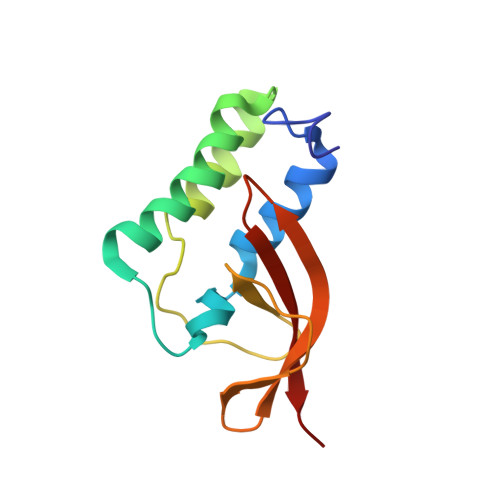
Structure and Genetic Analysis of the Arterivirus Nonstructural Protein 7{alpha}.
Manolaridis, I., Gaudin, C., Posthuma, C.C., Zevenhoven-Dobbe, J.C., Imbert, I., Canard, B., Kelly, G., Tucker, P.A., Conte, M.R., Snijder, E.J.(2011) J Virol 85: 7449-7453
- PubMed: 21561912
- DOI: https://doi.org/10.1128/JVI.00255-11
- Primary Citation of Related Structures:
2L8K - PubMed Abstract:
Arterivirus replicase polyproteins are cleaved into at least 13 mature nonstructural proteins (nsps), and in particular the nsp5-to-nsp8 region is subject to a complex processing cascade. The function of the largest subunit from this region, nsp7, which is further cleaved into nsp7α and nsp7β, is unknown. Using nuclear magnetic resonance (NMR) spectroscopy, we determined the solution structure of nsp7α of equine arteritis virus, revealing an interesting unique fold for this protein but thereby providing little clue to its possible functions. Nevertheless, structure-based reverse genetics studies established the importance of nsp7/nsp7α for viral RNA synthesis, thus providing a basis for future studies.
Organizational Affiliation:
European Molecular Biology Laboratory, Hamburg Outstation, D-22603 Hamburg, Germany.