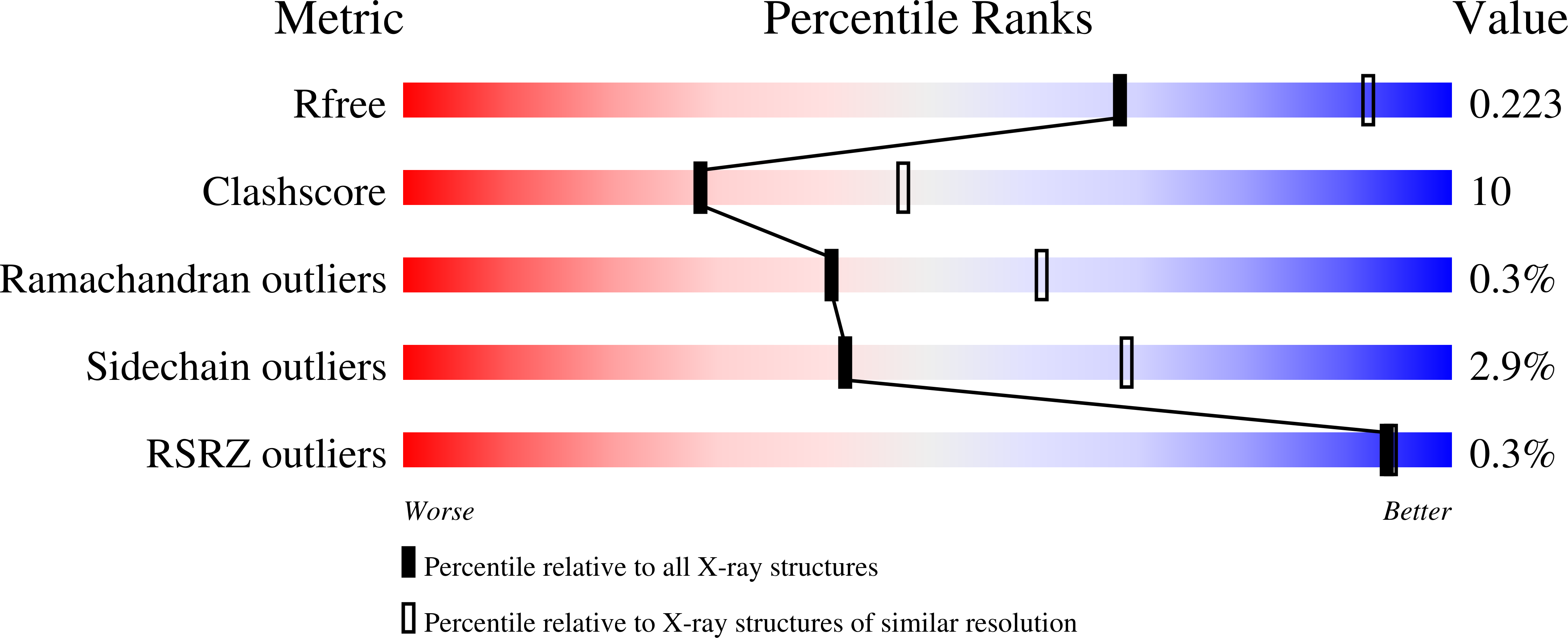
Invariant Thr(244) is essential for the efficient acylation step of the non-phosphorylating glyceraldehyde-3-phosphate dehydrogenase from Streptococcus mutans.
Pailot, A., D'ambrosio, K., Corbier, C., Talfournier, F., Branlant, G.(2006) Biochem J 400: 521-530
- PubMed: 16958622
- DOI: https://doi.org/10.1042/BJ20060843
- Primary Citation of Related Structures:
2ID2 - PubMed Abstract:
One of the most striking features of several X-ray structures of CoA-independent ALDHs (aldehyde dehydrogenases) in complex with NAD(P) is the conformational flexibility of the NMN moiety. However, the fact that the rate of the acylation step is high in GAPN (non-phosphorylating glyceraldehyde-3-phosphate dehydrogenase) from Streptococcus mutans implies an optimal positioning of the nicotinamide ring relative to the hemithioacetal intermediate within the ternary GAPN complex to allow an efficient and stereospecific hydride transfer. Substitutions of serine for invariant Thr244 and alanine for Lys178 result in a drastic decrease of the efficiency of hydride transfer which becomes rate-limiting. The crystal structure of the binary complex T244S GAPN-NADP shows that the absence of the beta-methyl group leads to a well-defined conformation of the NMN part, including the nicotinamide ring, clearly different from that depicted to be suitable for an efficient hydride transfer in the wild-type. The approximately 0.6-unit increase in pK(app) of the catalytic Cys302 observed in the ternary complex for both mutated GAPNs is likely to be due to a slight difference in positioning of the nicotinamide ring relative to Cys302 with respect to the wild-type ternary complex. Taken together, the data support a critical role of the Thr244 beta-methyl group, held in position through a hydrogen-bond interaction between the Thr244 beta-hydroxy group and the epsilon-amino group of Lys178, in permitting the nicotinamide ring to adopt a conformation suitable for an efficient hydride transfer during the acylation step for all the members of the CoA-independent ALDH family.
Organizational Affiliation:
MAEM, UMR 7567 Nancy-Université, CNRS, Faculté des Sciences, 54506 Vandoeuvre Cedex, France.