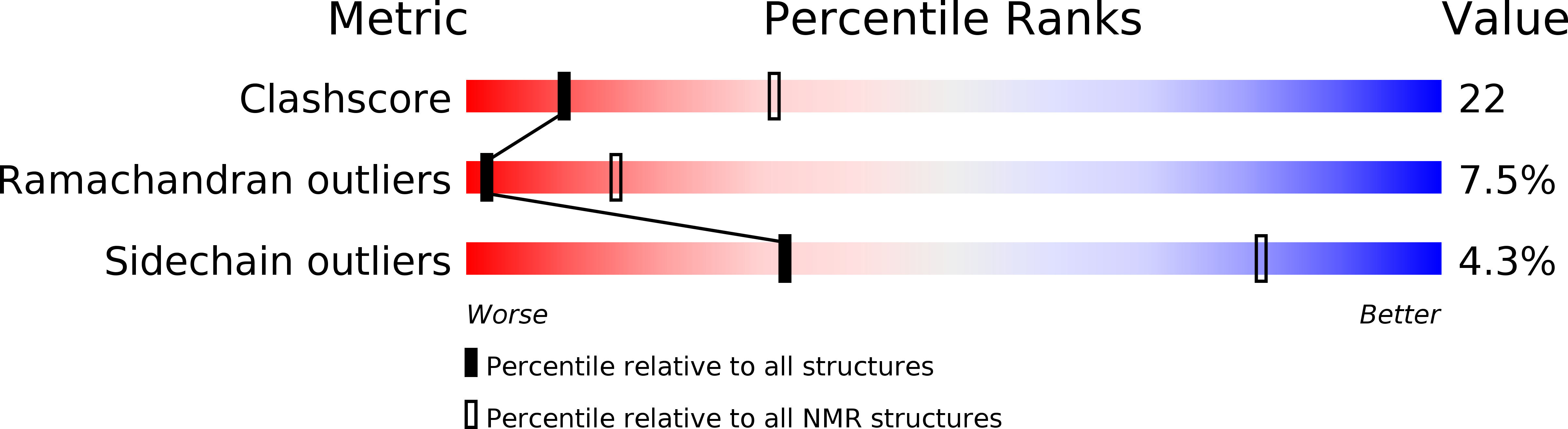Structure of the Chromo Barrel Domain from the Mof Acetyltransferase
Nielsen, P.R., Nietlispach, D., Buscaino, A., Warner, R.J., Akhtar, A., Murzin, A.G., Murzina, N.V., Laue, E.D.(2005) J Biol Chem 280: 32326
- PubMed: 15964847
- DOI: https://doi.org/10.1074/jbc.M501347200
- Primary Citation of Related Structures:
2BUD - PubMed Abstract:
We report here the structure of the putative chromo domain from MOF, a member of the MYST family of histone acetyltransferases that acetylates histone H4 at Lys-16 and is part of the dosage compensation complex in Drosophila. We found that the structure of this domain is a beta-barrel that is distinct from the alpha + beta fold of the canonical chromo domain. Despite the differences, there are similarities that support an evolutionary relationship between the two domains, and we propose the name "chromo barrel." The chromo barrel domains may be divided into two groups, MSL3-like and MOF-like, on the basis of whether a group of conserved aromatic residues is present or not. The structure suggests that, although the MOF-like domains may have a role in RNA binding, the MSL3-like domains could instead bind methylated residues. The MOF chromo barrel shares a common fold with other chromatin-associated modules, including the MBT-like repeat, Tudor, and PWWP domains. This structural similarity suggests a probable evolutionary pathway from these other modules to the canonical chromo domains (or vice versa) with the chromo barrel domain representing an intermediate structure.
Organizational Affiliation:
Cambridge Centre for Molecular Recognition, Department of Biochemistry, University of Cambridge, UK.