Crystal Structure of Proaerolysin at 2.3 A Resolution and Structural Analyses of Single-site Mutants as a Basis for Understanding Membrane Insertion of the Toxin
Parker, M.W., Feil, S.C., Tang, J.W.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Aerolysin | 470 | Aeromonas hydrophila | Mutation(s): 1 | 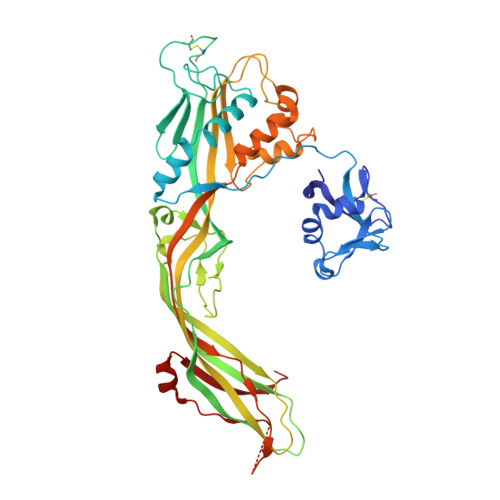 | |
UniProt | |||||
Find proteins for P09167 (Aeromonas hydrophila) Explore P09167 Go to UniProtKB: P09167 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P09167 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 70.711 | α = 90 |
| b = 91.141 | β = 90 |
| c = 168.215 | γ = 90 |
| Software Name | Purpose |
|---|---|
| DENZO | data reduction |
| SCALEPACK | data scaling |
| AMoRE | phasing |
| CNS | refinement |