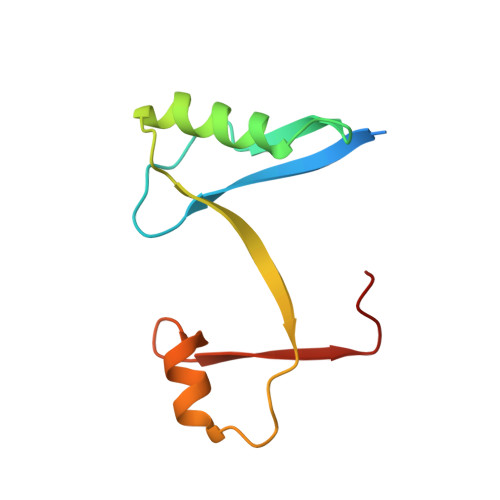
The crystal structure of plant ATG12 and its biological implication in autophagy.
Suzuki, N.N., Yoshimoto, K., Fujioka, Y., Ohsumi, Y., Inagaki, F.(2005) Autophagy 1: 119-126
- PubMed: 16874047
- DOI: https://doi.org/10.4161/auto.1.2.1859
- Primary Citation of Related Structures:
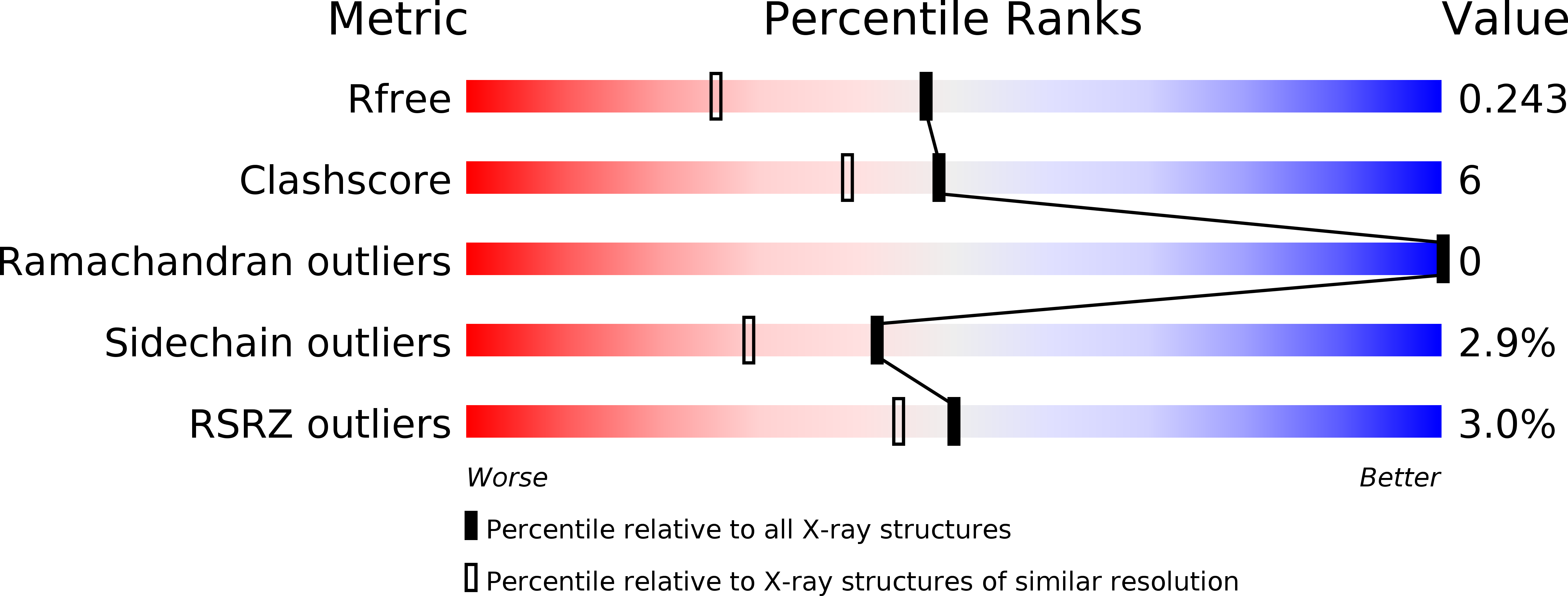
1WZ3 - PubMed Abstract:
Atg12 is a post-translational modifier that is activated and conjugated to its single target, Atg5, by a ubiquitin-like conjugation system. The Atg12-Atg5 conjugate is essential for autophagy, the bulk degradation process of cytoplasmic components by the vacuolar/lysosomal system. Here, we demonstrate that the Atg12 conjugation system exists in Arabidopsis and is essential for plant autophagy as well as in yeast and mammals. We also report the crystal structure of Arabidopsis thaliana (At) ATG12 at 1.8 A resolution. Despite no obvious sequence homology with ubiquitin, the structure of AtATG12 shows a ubiquitin fold strikingly similar to those of mammalian homologs of Atg8, the other ubiquitin-like modifier essential for autophagy, which is conjugated to phosphatidylethanolamine. Two types of hydrophobic patches are present on the surface of AtATG12: one is conserved in both Atg12 and Atg8 orthologs, while the other is unique to Atg12 orthologs. Considering that they share Atg7 as an E1-like enzyme, we suggest that the first hydrophobic patch is responsible for the conjugation reaction, while the latter is involved in Atg12-specific functions.
Organizational Affiliation:
Department of Structural Biology, Graduate School of Pharmaceutical Sciences, Hokkaido University, Kita-ku, Sapporo, Japan.