Structural evidence for guanidine-protein side chain interactions: crystal structure of CutA from Pyrococcus horikoshii in 3M guanidine hydrochloride
Tanaka, Y., Tsumoto, K., Umetsu, M., Nakanishi, T., Yasutake, Y., Sakai, N., Yao, M., Tanaka, I., Arakawa, T., Kumagai, I.(2004) Biochem Biophys Res Commun 323: 185-191
- PubMed: 15351719
- DOI: https://doi.org/10.1016/j.bbrc.2004.08.081
- Primary Citation of Related Structures:
1UMJ - PubMed Abstract:
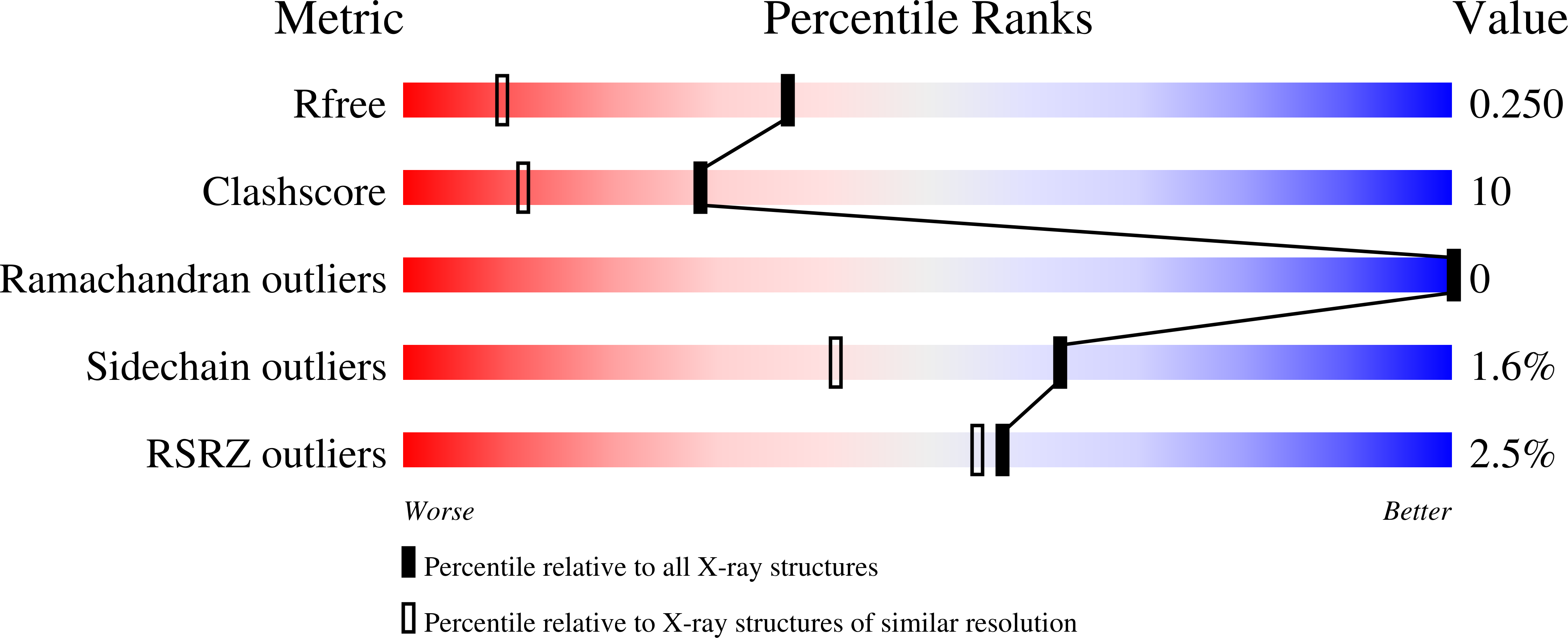
This study was carried out to investigate the structural perturbation of the protein's local structure by the denaturants under non-denaturing conditions. Crystal structure of CutA from an archaeon Pyrococcus horikosii (PhoCutA), a heavy-metal binding protein, was determined at 1.6-angstroms resolution in the presence of 3 M guanidine HCl (GdnHCl). Native PhoCutA has a large number of short intramolecular hydrogen bonds and salt bridges on the protein surface, of which greater than 90% of hydrogen bonds and all salt bridges were retained in 3 M GdnHCl. Hydrogen bonds that disappeared in the GdnHCl crystal structure were mainly located on the protein surface, especially around the structurally perturbed loop, suggesting interactions between peptide groups and GdnHCl. Only a few GdnH+ ions were observed in the crystal structure, although none at the surface, of the protein. Two GdnH+ ions were observed in the center of the trimeric structure, replacing water molecules, and were hydrogen bonded with Asp84 and Asp86 of each chain. The exterior loop from Tyr39 to Lys44, including Trp40-Trp41, was perturbed structurally. Decreases in temperature factors were observed in beta strand 5 and the N terminus of helix 3. These results suggest the specific bindings of GdnH+ with some acidic residues and the non-specific bindings around Trp residues and peptide groups on the protein surface and that binding of GdnHCl to the native protein is limited, resulting in local structural perturbation.
Organizational Affiliation:
Department of Biomolecular Engineering, Graduate School of Engineering, Tohoku University, Aobayama 07, Aoba-ku, Sendai 980-8579, Japan.