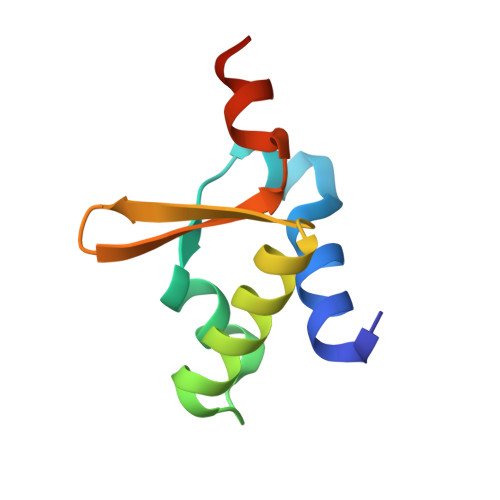
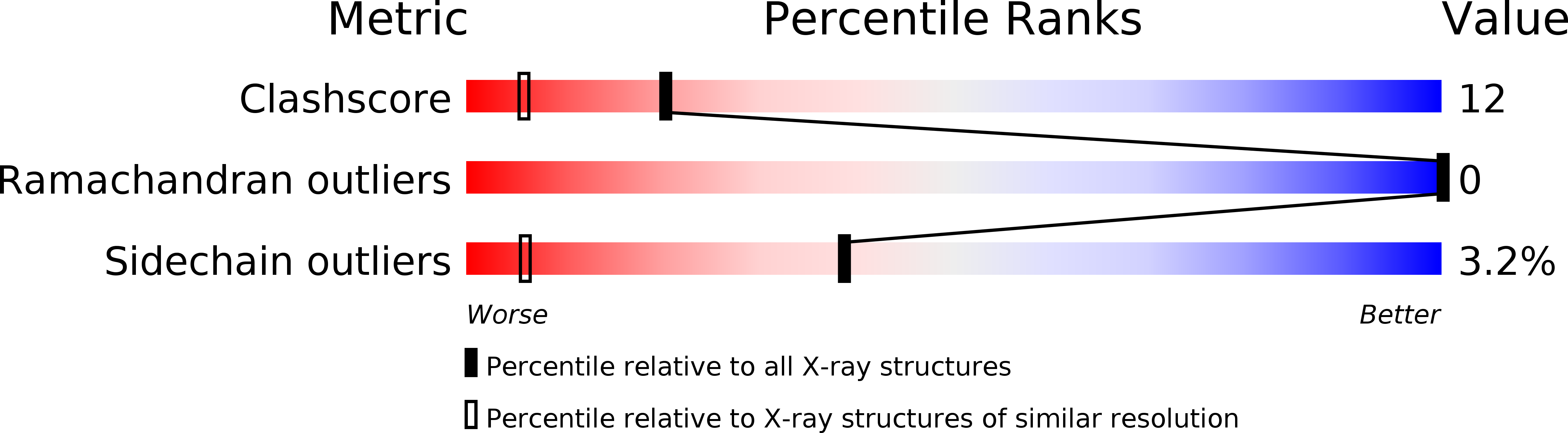Crystal Structure of Dissimilatory Sulfite Reductase D (DsrD) Protein-Possible Interaction with B- and Z-DNA by Its Winged-Helix Motif
Mizuno, N., Voordouw, G., Miki, K., Sarai, A., Higuchi, Y.(2003) Structure 11: 1133-1140
- PubMed: 12962631
- DOI: https://doi.org/10.1016/s0969-2126(03)00156-4
- Primary Citation of Related Structures:
1UCR - PubMed Abstract:
The crystal structure of DsrD from Desulfovibrio vulgaris Hildenborough has been determined at 1.2 A resolution. DsrD is in a dimeric form in the crystal, and five sulfate anions were located on the surface. The structure of DsrD comprises a winged-helix motif, which shows the highest structural homology to similar motifs found in Z-DNA binding proteins and some B-DNA binding proteins. The core structure of the molecule is constructed by intramolecular interactions of hydrophobic residues, which are well conserved in DNA binding proteins, suggesting that these proteins belong to the same superfamily on the basis of the structure. These results indicate a possible role of DsrD in transcription or translation of genes for enzymes catalyzing dissimilatory sulfite reduction.
Organizational Affiliation:
Division of Chemistry, Graduate School of Science, Kyoto University, Sakyo, Kyoto 606-8502, Japan.