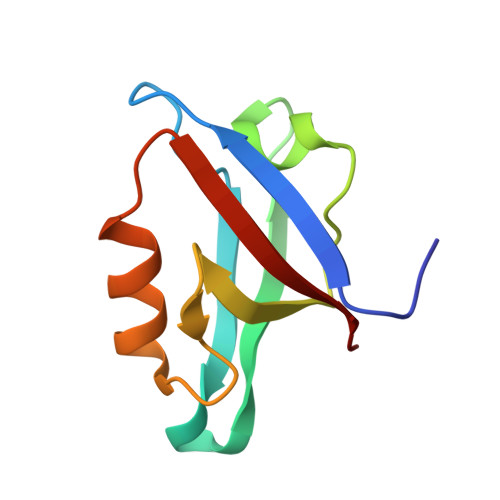
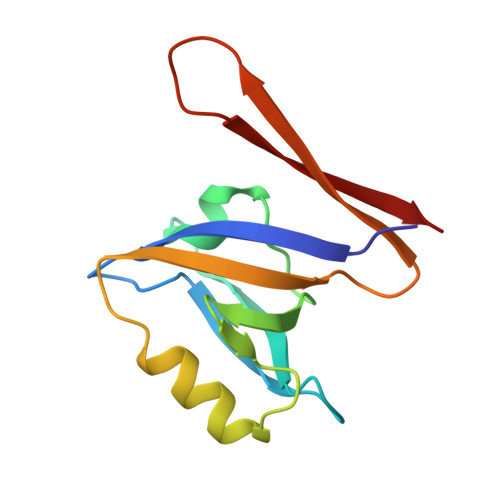
Unexpected modes of PDZ domain scaffolding revealed by structure of nNOS-syntrophin complex.
Hillier, B.J., Christopherson, K.S., Prehoda, K.E., Bredt, D.S., Lim, W.A.(1999) Science 284: 812-815
- PubMed: 10221915
- Primary Citation of Related Structures:
1QAU, 1QAV - PubMed Abstract:
The PDZ protein interaction domain of neuronal nitric oxide synthase (nNOS) can heterodimerize with the PDZ domains of postsynaptic density protein 95 and syntrophin through interactions that are not mediated by recognition of a typical carboxyl-terminal motif. The nNOS-syntrophin PDZ complex structure revealed that the domains interact in an unusual linear head-to-tail arrangement. The nNOS PDZ domain has two opposite interaction surfaces-one face has the canonical peptide binding groove, whereas the other has a beta-hairpin "finger." This nNOS beta finger docks in the syntrophin peptide binding groove, mimicking a peptide ligand, except that a sharp beta turn replaces the normally required carboxyl terminus. This structure explains how PDZ domains can participate in diverse interaction modes to assemble protein networks.
Organizational Affiliation:
Department of Cellular and Molecular Pharmacology, University of California, San Francisco, San Francisco, CA 94143, USA.