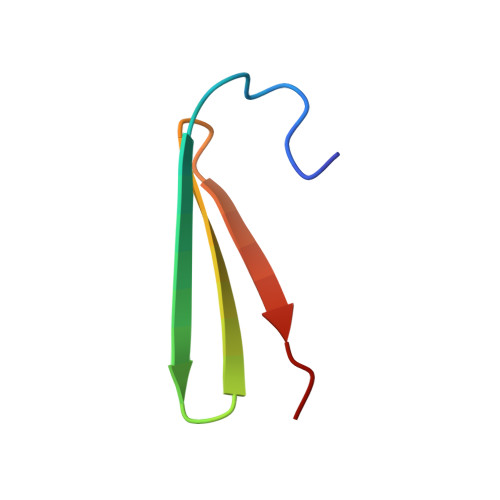
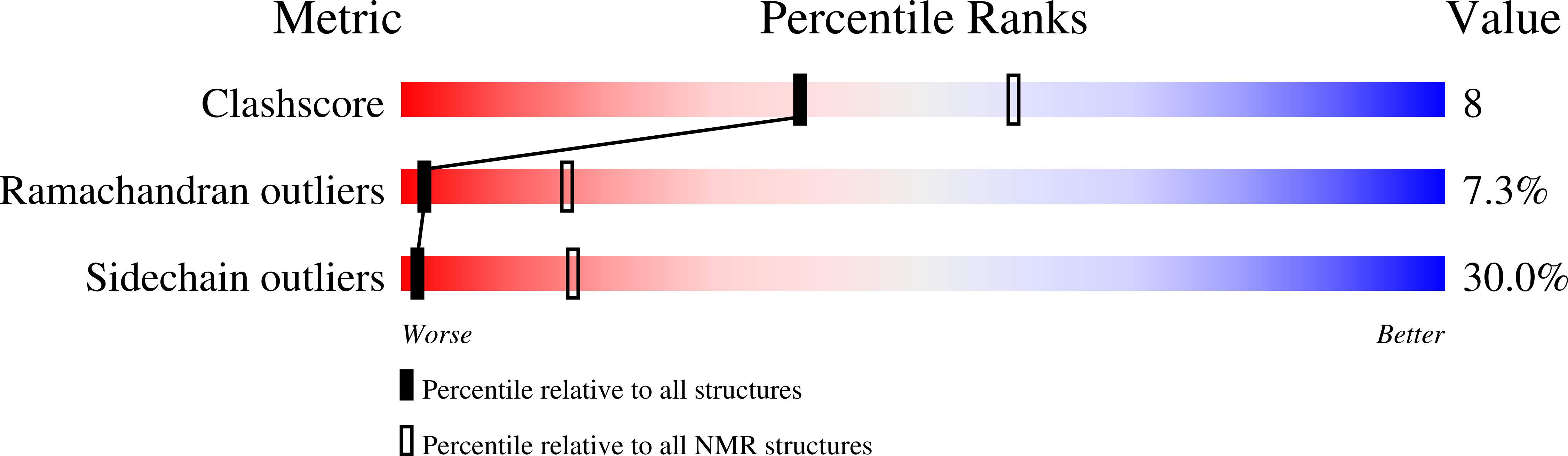Structure of the beta Subunit of Translation Initiation Factor 2 from the Archaeon Methanococcus jannaschii: A Representative of the eIF2beta/eIF5 Family of Proteins
Cho, S., Hoffman, D.W.(2002) Biochemistry 41: 5730-5742
- PubMed: 11980477
- DOI: https://doi.org/10.1021/bi011984n
- Primary Citation of Related Structures:
1K81, 1K8B - PubMed Abstract:
The beta subunit of archaeal translation initiation factor 2 (aIF2beta) is a representative of a family of proteins whose members include the beta subunit of eukaryotic translation initiation factor 2 (eIF2beta) and the N-terminal domain within translation initiation factor 5 (eIF5); no members of this family of proteins have been structurally characterized up to this time. In the work presented here, aIF2beta from Methanococcus jannaschii was expressed in Escherichia coli, purified, and analyzed using multidimensional NMR methods. The aIF2beta was found to contain two independent structural domains. The N-terminal domain contains a four-stranded antiparallel beta sheet and two alpha helices, and is structurally similar to the DNA-binding domain of a yeast heat shock transcription factor and a domain within ribosomal protein S4. This structural similarity was an unanticipated result, since no significant homology was detected at the level of primary sequence. The C-terminal domain of aIF2beta contains a zinc-binding motif of three antiparallel beta strands, with four conserved cysteines arranged as two CXXC units separated by 17 residues. Conserved residues on the surface of each domain that are likely candidates for direct interaction with other components of the translational apparatus were identified. The significant primary sequence homology between archaeal aIF2beta and the eukaryotic eIF2beta and eIF5, when combined with the structural results in the work presented here, permitted structural features to be predicted for these latter two eukaryotic proteins.
Organizational Affiliation:
Department of Chemistry and Biochemistry, Institute for Cellular and Molecular Biology, University of Texas at Austin, Austin, Texas 78712, USA.