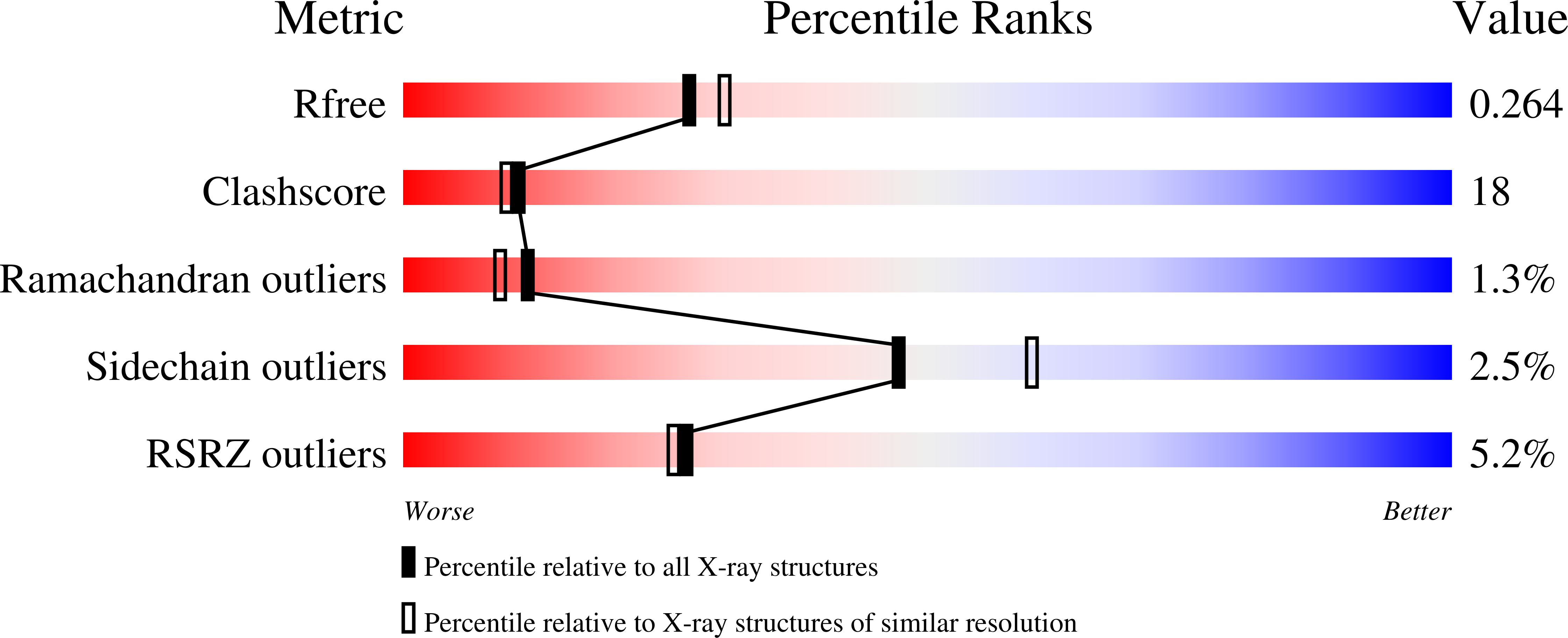
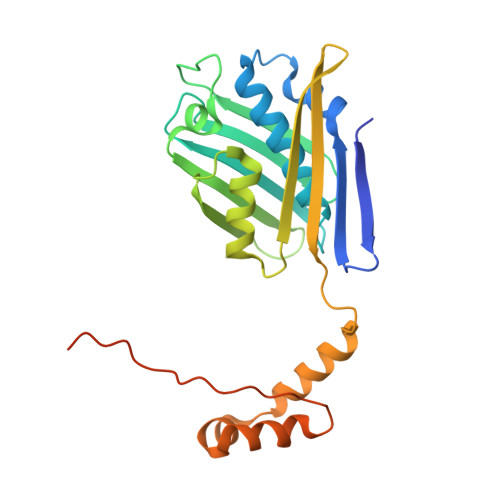
The 2.2 A crystal structure of Hsp33: a heat shock protein with redox-regulated chaperone activity.
Vijayalakshmi, J., Mukhergee, M.K., Graumann, J., Jakob, U., Saper, M.A.(2001) Structure 9: 367-375
- PubMed: 11377197
- DOI: https://doi.org/10.1016/s0969-2126(01)00597-4
- Primary Citation of Related Structures:
1HW7 - PubMed Abstract:
One strategy that cells employ to respond to environmental stresses (temperature, oxidation, and pathogens) is to increase the expression of heat shock proteins necessary to maintain viability. Several heat shock proteins function as molecular chaperones by binding unfolded polypeptides and preventing their irreversible aggregation. Hsp33, a highly conserved bacterial heat shock protein, is a redox-regulated molecular chaperone that appears to protect cells against the lethal effects of oxidative stress.
Organizational Affiliation:
Biophysics Research Division, University of Michigan, 48109, Ann Arbor, MI, USA.