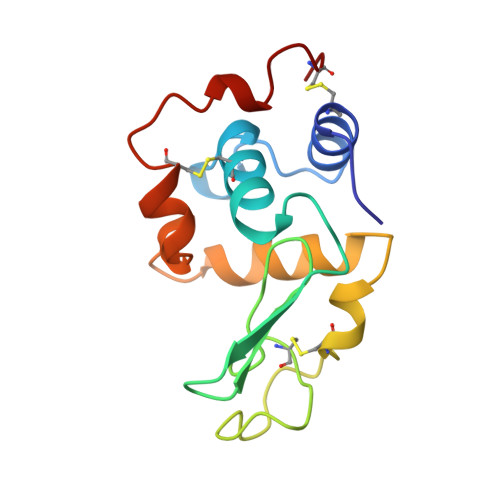
Structure of a glutathionylated human lysozyme: a folding intermediate mimic in the formation of a disulfide bond.
Inaka, K., Miki, K., Kikuchi, M., Taniyama, Y., Matsushima, M.(1995) Acta Crystallogr D Biol Crystallogr 51: 619-625
- PubMed: 15299791
- DOI: https://doi.org/10.1107/S0907444994013478
- Primary Citation of Related Structures:
1HNL - PubMed Abstract:
The three-dimensional structure of a mutant human lysozyme, C77A-a, in which the residue Cys77 is replaced by alanine, has been refined to an R value of 0.125 using 8230 reflections in the resolution range 10.0-1.8 A. It has been shown that C77A-a, in which the counterpart of Cys77 (Cys95) is modified with glutathione, has been shown to mimic an intermediate in the formation of the disulfide bond Cys77-Cys95 during the folding of human lysozyme [Hayano, Inaka, Otsu, Taniyama, Miki, Matsushima & Kikuchi (1993). FEBS Lett. 328, 203-208]. An earlier structure demonstrates that its overall structure is essentially identical to that of the wild-type protein and served as the starting model. The refined model includes atoms for all protein residues (1-130), 20 glutathione atoms and 113 water atoms. Further refinement shows more clearly the details of the protein, the bound glutathione molecule and solvent structure. However, the main-chain folding and the atomic thermal factors of the loop region from Thr70 to Leu79 were highly affected by the binding of the glutathione molecule, as compared with those of the wild-type protein. The bound glutathione shifted the main-chain atoms from Va174 to Ala77 by more than 6.0 A, and the temperature factors of the atoms in the loop region were quite high (more than 40 A(2)), indicating that the backbone conformation of this region is highly flexible and that the loop region is not folded in the specific conformation observed in the wild-type protein. These results strongly suggest that the loop structure in human lysozyme is folded later than the other regions of the protein in vivo, as observed in in vitro folding. Since the bound glutathione is efficiently and irreversibly dissociated by protein disulfide isomerase, the glutathione molecule may act as a protecting group to prevent the formation of an incorrect disulfide bond in the protein folding process in vivo.
Organizational Affiliation:
Protein Engineering Research Institute, Suita, Osaka, Japan.