Crystal Structure of the Di-Haemcytochrome C Peroxidase from Pseudomonas Aeruginosa
Fulop, V., Ridout, C.J., Greenwood, C., Hajdu, J.(1995) Structure 3: 1225
- PubMed: 8591033
- DOI: https://doi.org/10.1016/s0969-2126(01)00258-1
- Primary Citation of Related Structures:
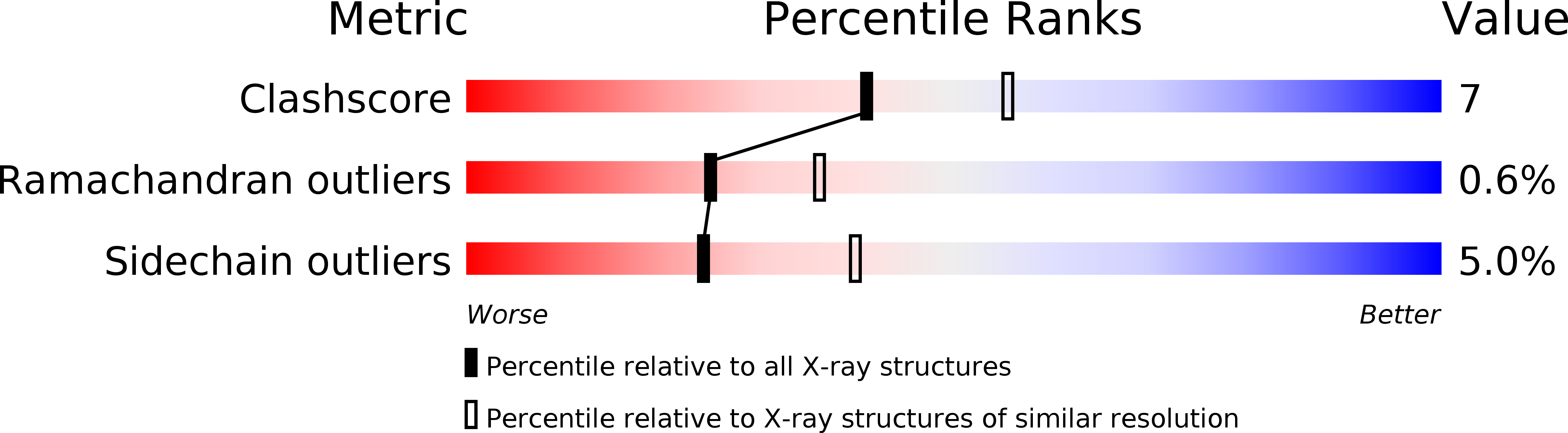
1EB7 - PubMed Abstract:
Cytochrome c peroxidase from Pseudomonas aeruginosa (PsCCP) represents a new class of peroxidases which work without the need to create a semi-stable free radical for catalysis. The enzyme is located in the bacterial periplasm where its likely function is to provide protection against toxic peroxides. The soluble 323-residue single polypeptide chain contains two covalent c-type haems with very different properties: one of them is a low-potential (-330 mV) centre where hydrogen peroxide is reduced (the peroxidatic site); the other is a high-potential (+320 mV) centre which feeds electrons to the peroxidatic site from soluble electron-shuttle proteins such as cytochrome c and azurin. The crystal structure of the oxidized form of PsCCP has been determined to 2.4 A resolution by multiple isomorphous replacement, and refined to an R-factor of 19.2%. PsCCP is organized into two domains, both of them containing a covalent c-haem in a structure reminiscent of class 1 cytochromes c. The domains are related by a quasi-twofold axis. The domain interface holds a newly discovered calcium-binding site with an unusual set of ligands. The likely function of the calcium site is to maintain the structural integrity of the enzyme and/or to modulate electron transfer between the two haem domains. The low-potential haem has two histidine axial ligands (His55 and His71) and the high-potential haem is ligated by His201 and Met275. There are no polar residues at the peroxidatic site in the inactive oxidized enzyme. The structure suggests that, in the half-reduced functional form of the enzyme, the low-potential haem has to shed His71 in order to make the enzyme catalytically competent. This process is likely to trigger a reorganization of the active site, and may introduce a new residues into the haem pocket.
Organizational Affiliation:
Laboratory of Molecular Biophysics, University of Oxford, UK.