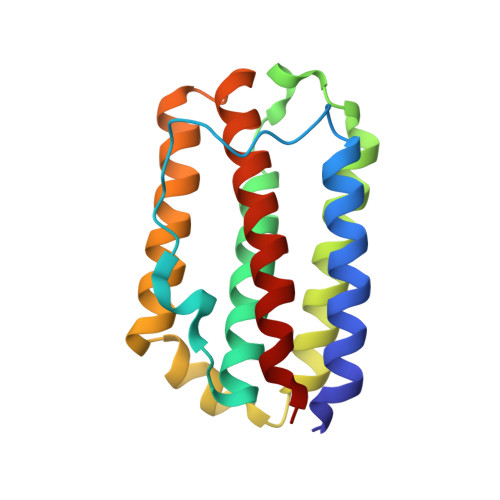
Refined crystal structure of recombinant murine interferon-beta at 2.15 A resolution
Senda, T., Saitoh, S., Mitsui, Y.(1995) J Mol Biol 253: 187-207
- PubMed: 7473712
- DOI: https://doi.org/10.1006/jmbi.1995.0544
- Primary Citation of Related Structures:
1WU3 - PubMed Abstract:
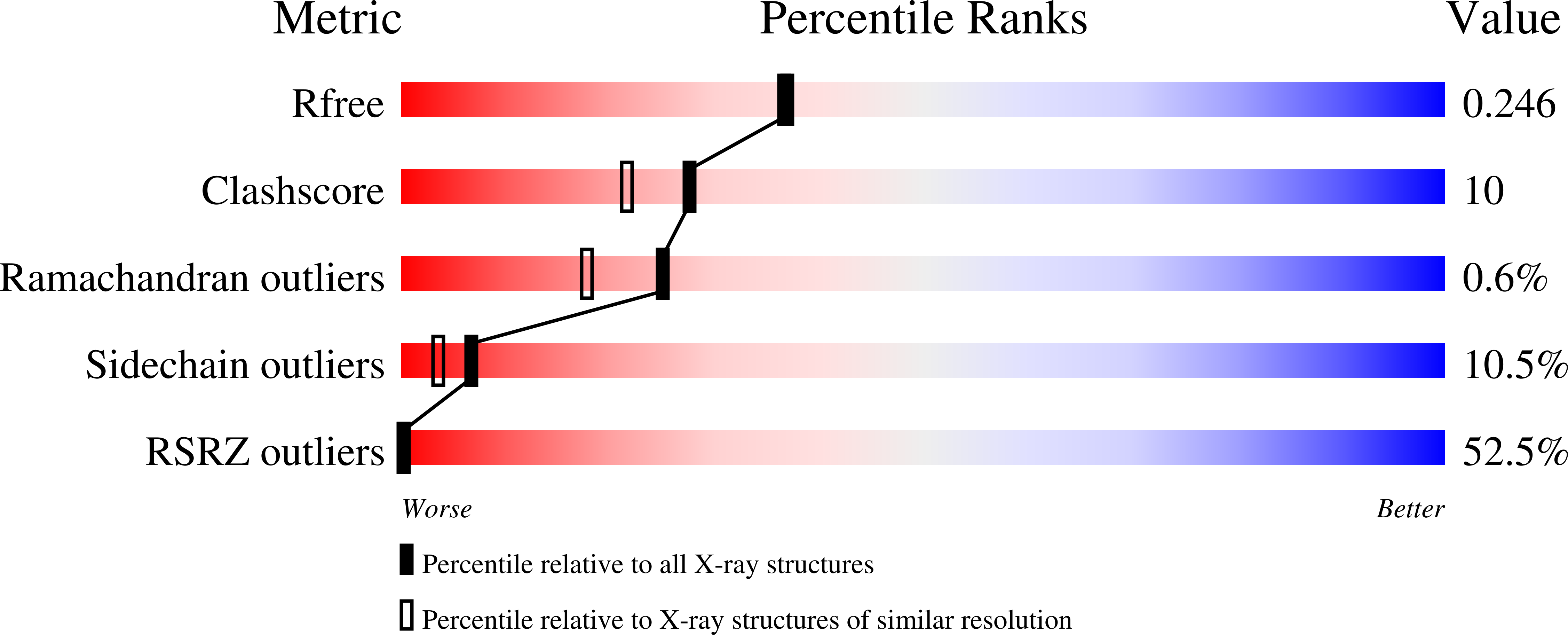
The crystal structure of recombinant murine interferon-beta (reMuIFN-beta) has been refined at 2.15 A resolution using newly collected synchrotron data. Based on 11,228 reflections (8.0 to 2.15 A), a final R-factor of 19.1% (with a free R-factor of 25.8%) was obtained with a model obeying standard geometry within 0.013 A in bond lengths and 1.4 degrees in bond angles. Compared with the previously reported model, several amino acid residues in helix A are frame-shifted, the conformations are changed for parts of loops AB and BC, helix C is extended and a new short helix exists in loop CD. Evolutionary considerations taken together, the type I interferons appear to share common structural features with respect to the chain-folding topology and the hydrogen-bond networks between various polypeptide segments. Specifically, the disposition of the C-terminal segment of loop AB (after Arg33), known to be an important receptor-binding site, seems to be strictly maintained among the type I interferons. The exposed amino acid residues on helices A and C, which have recently been implicated as the binding site for another receptor molecule, are less well conserved. This may be responsible for varied cellular effects among the subtypes of type I interferons.
Organizational Affiliation:
Department of BioEngineering, Nagaoka University of Technology, Niigata, Japan.