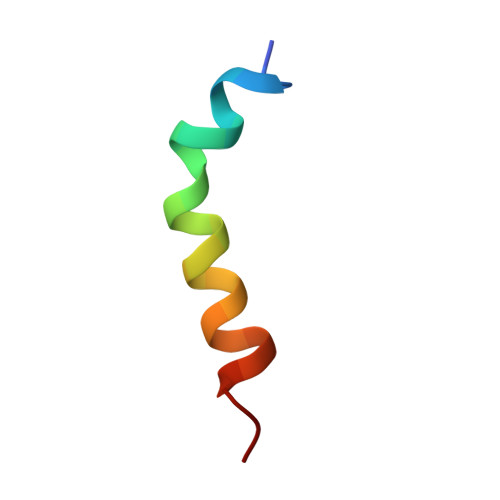
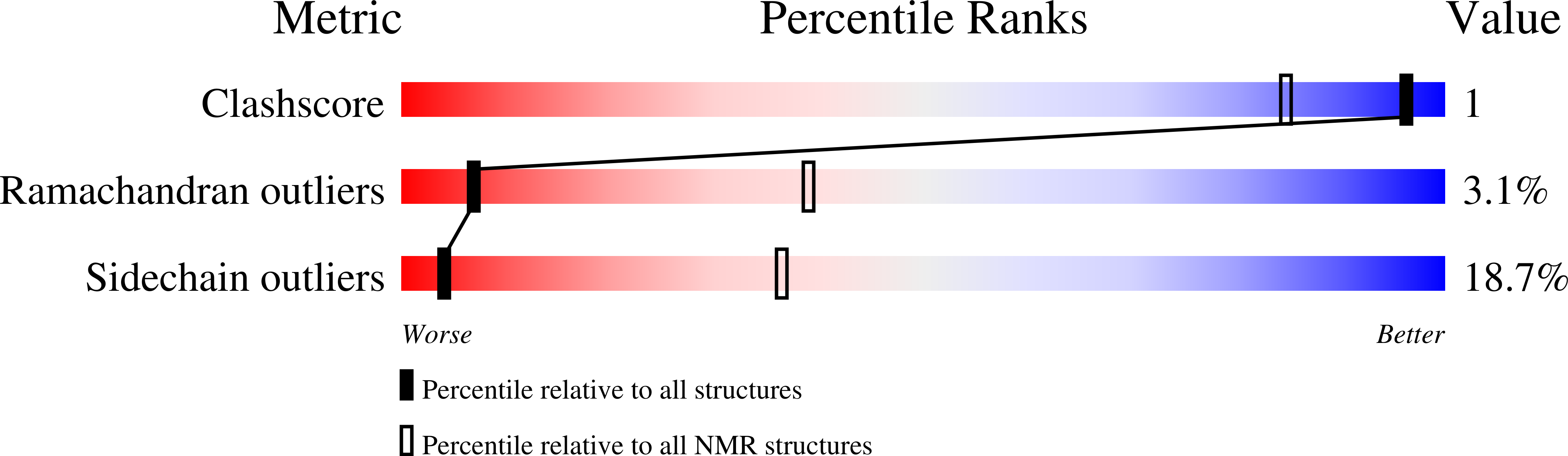Synthesis and structure determination by NMR of a putative vacuolar targeting peptide and model of a proteinase inhibitor from Nicotiana alata.
Nielsen, K.J., Hill, J.M., Anderson, M.A., Craik, D.J.(1996) Biochemistry 35: 369-378
- PubMed: 8555206
- DOI: https://doi.org/10.1021/bi952228i
- Primary Citation of Related Structures:
1VTP - PubMed Abstract:
NA-proPI is a 40.3-kDa multidomain precursor protein found in the stigma of the ornamental tobacco Nicotiana alata. It is selectively targeted to the vacuole and, as the plant matures, is processed to produce a series of five 6-kDa proteinase inhibitors (one chymotrypsin and four trypsin reactive sites) which are thought to play a vital role in plant protection against insect pests. A putative sixth domain with a chymotrypsin reactive site is likely to be formed by three disulfide bridges linking the N- and C-terminal fragments of NA-proPI. This domain contains two distinct structural elements: a 54-residue sequence with high identity to each of the five repeated PI domains, and a nonrepeated 25-residue sequence at the C-terminus which is proposed to contain a vacuolar targeting peptide. The structure of the putative sixth domain was predicted using a combination of secondary structure prediction and homology modeling based on the known structure of one of the intact domains. A 26-residue peptide corresponding to the nonrepeated C-terminal sequence and encompassing the putative vacuolar targeting sequence was synthesized and its structure determined using 1H NMR spectroscopy and simulated annealing calculations. The peptide was found to adopt an amphipathic helical structure. The calculations based on NOE data suggested that the helix is curved, with a hydrophobic concave face and a hydrophilic convex face. This curvature is consistent with an observed periodicity in backbone NH chemical shifts. The structure of the entire sixth domain was modeled by combining the experimentally determined structure of the putative vacuolar targeting peptide with the homology model of the PI domain. In this model the alpha-helix of the putative targeting peptide protrudes from the otherwise compact PI domain. This observation is consistent with the requirement for targeting sequences to be relatively exposed for recognition by the sorting apparatus. As there is no consensus on the structure of vacuolar targeting sequences, this study provides a valuable insight into their potential mechanism of interaction with the cellular sorting apparatus.
Organizational Affiliation:
Centre for Drug Design and Development, University of Queensland, St. Lucia, Australia.