Allele-Dependent Similarity between Viral and Self-Peptide Presentation by Hla-B27 Subtypes
Fiorillo, M.T., Ruckert, C., Hulsmeyer, M., Sorrentino, R., Saenger, W., Ziegler, A., Uchanska-Ziegler, B.(2005) J Biol Chem 280: 2962
- PubMed: 15537660
- DOI: https://doi.org/10.1074/jbc.M410807200
- Primary Citation of Related Structures:
1UXS, 1UXW - PubMed Abstract:
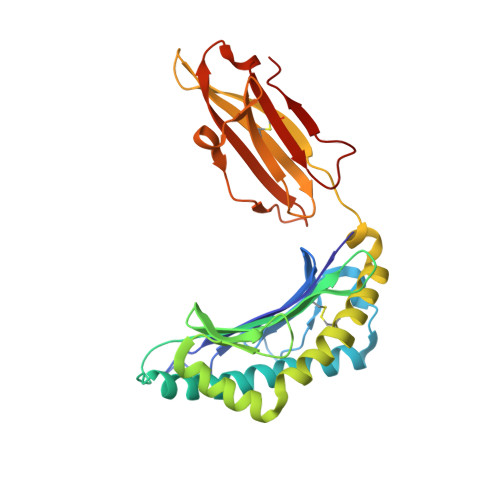
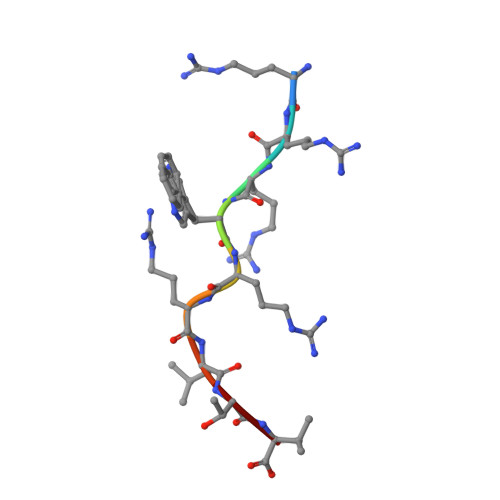
Molecular mimicry is discussed as a possible mechanism that may contribute to the development of autoimmune diseases. It could also be involved in the differential association of the human major histocompatibility subtypes HLA-B(*)2705 and HLA-B(*)2709 with ankylosing spondylitis. These two subtypes differ only in residue 116 of the heavy chain (Asp in B(*)2705 and His in B(*)2709), but the reason for the differential disease association is not understood. Using x-ray crystallography, we show here that the viral peptide pLMP2 (RRRWRRLTV, derived from latent membrane protein 2 (residues 236-244) of Epstein-Barr virus) is presented by the B(*)2705 and B(*)2709 molecules in two drastically deviating conformations. Extensive structural similarity between pLMP2 and the self-peptide pVIPR (RRKWRRWHL, derived from vasoactive intestinal peptide type 1 receptor (residues 400-408)) is observed only when the peptides are presented by B(*)2705 because of a salt bridge between Arg(5) of both peptides and the subtype-specific heavy chain residue Asp(116). Combined with functional studies using pLMP2/pVIPR-cross-reactive cytotoxic T cell lines and clones, together with target cells presenting these peptides or a modified peptide analogue, our results reveal that a pathogen-derived peptide can exhibit major histocompatibility complex class I subtype-dependent, drastically distinct binding modes. Furthermore, the results demonstrate that molecular mimicry between pLMP2 and pVIPR in the HLA-B27 context is an allele-dependent property.
Organizational Affiliation:
Dipartimento di Biologia Cellulare e dello Sviluppo, Università di Roma La Sapienza, via dei Sardi 70, 00185 Roma, Italy.