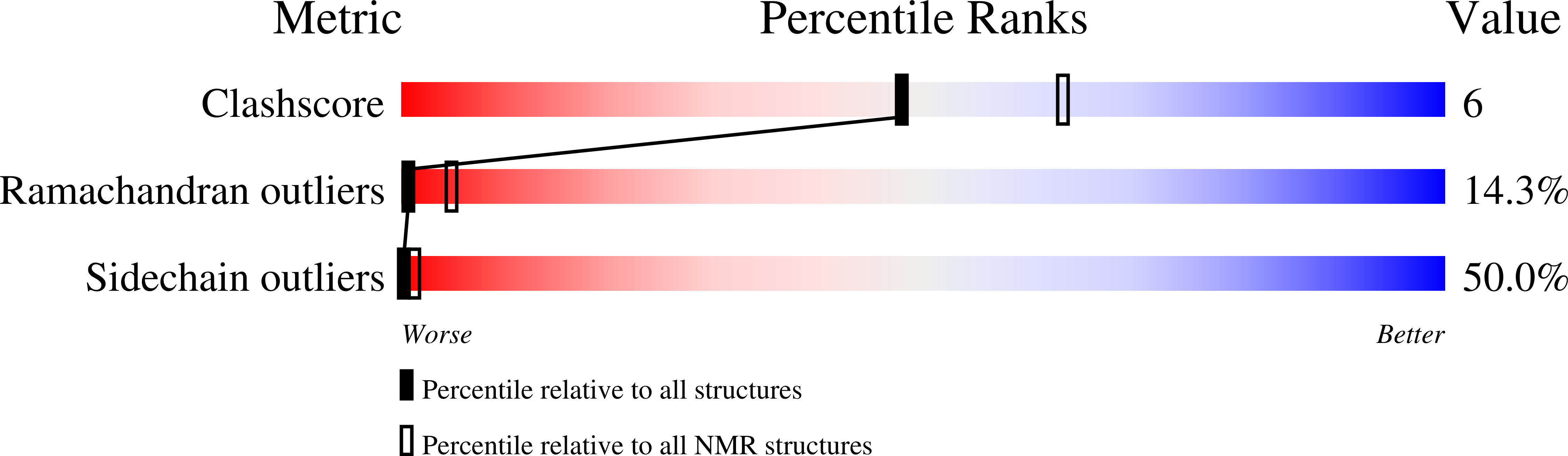Cannabinoid receptor-G protein interactions: G(alphai1)-bound structures of IC3 and a mutant with altered G protein specificity.
Ulfers, A.L., McMurry, J.L., Miller, A., Wang, L., Kendall, D.A., Mierke, D.F.(2002) Protein Sci 11: 2526-2531
- PubMed: 12237474
- DOI: https://doi.org/10.1110/ps.0218402
- Primary Citation of Related Structures:
1LVQ, 1LVR - PubMed Abstract:
The structure of the C-terminal region of the third cytoplasmic loop (IC3) of the cannabinoid receptor one (CB1) bound to G(alphai1) has been determined using transferred nuclear Overhauser effects (NOEs). The wild-type IC3 sequence is helical when associated with G(alphai1). In contrast, a peptide containing the amino-acid inversion, Ala(341)-Leu(342) adopts a single turn. These findings correlate with the attenuated G(i) association of CB1 with the Ala(341)-Leu(342) mutation previously observed in vivo and the diminished stimulation of G(alphai1) GTPase activity by the corresponding peptide demonstrated in vitro here. These results, the first to report the structure of a GPCR domain while associated with G protein, imply the C-terminus of CB1 IC3, a region with high-sequence conservation among G-protein coupled receptors, must be helical for efficient coupling and activation of the G(i) protein.
Organizational Affiliation:
Department of Molecular Pharmacology, Division of Biology and Medicine, Brown University, Providence, Rhode Island 02912, USA.