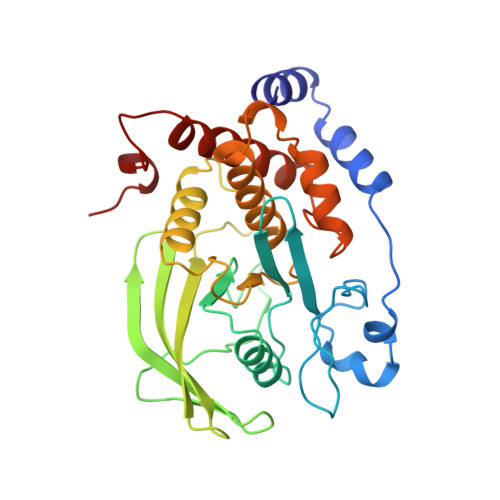
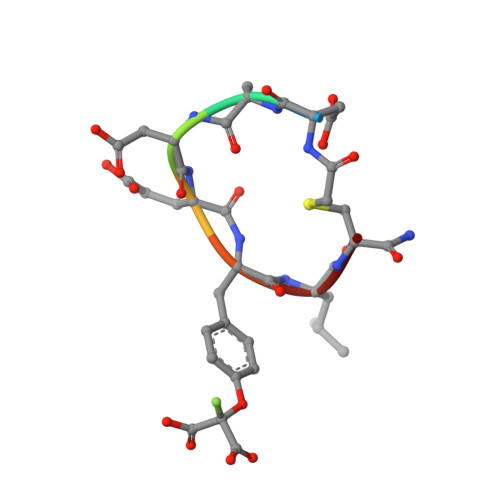
Structural basis for inhibition of the protein tyrosine phosphatase 1B by phosphotyrosine peptide mimetics.
Groves, M.R., Yao, Z.J., Roller, P.P., Burke Jr., T.R., Barford, D.(1998) Biochemistry 37: 17773-17783
- PubMed: 9922143
- DOI: https://doi.org/10.1021/bi9816958
- Primary Citation of Related Structures:
1BZC, 1BZH, 1BZJ - PubMed Abstract:
Protein tyrosine phosphatases regulate diverse cellular processes and represent important targets for therapeutic intervention in a number of diseases. The crystal structures of protein tyrosine phosphatase 1B (PTP1B) in complex with small molecule inhibitors based upon two classes of phosphotyrosine mimetics, the (difluoronaphthylmethyl)phosphonic acids and the fluoromalonyl tyrosines, have been determined to resolutions greater than 2.3 A. The fluoromalonyl tyrosine residue was incorporated within a cyclic hexapeptide modeled on an autophosphorylation site of the epidermal growth factor receptor. The structure of this inhibitor bound to PTP1B represents the first crystal structure of a non-phosphonate-containing inhibitor and reveals the mechanism of phosphotyrosine mimicry by the fluoromalonyl tyrosine residue and the nature of its interactions within the catalytic site of PTP1B. In contrast to complexes of PTP1B with phosphotyrosine-containing peptides, binding of the fluoromalonyl tyrosine residue to the catalytic site of PTP1B is not accompanied by closure of the catalytic site WPD loop. Structures of PTP1B in complex with the (difluoronaphthylmethyl)phosphonic acid derivatives reveal that substitutions of the naphthalene ring modulate the mode of inhibitor binding to the catalytic site and provide the potential for enhanced inhibitor affinity and the generation of PTP-specific inhibitors. These results provide a framework for the rational design of higher affinity and more specific phosphotyrosine mimetic inhibitors of not only protein tyrosine phosphatases but also SH2 and PTB domains.
Organizational Affiliation:
Department of Biochemistry, University of Oxford, United Kingdom.