Structures of the HIV-1 capsid protein dimerization domain at 2.6 A resolution.
Worthylake, D.K., Wang, H., Yoo, S., Sundquist, W.I., Hill, C.P.(1999) Acta Crystallogr D Biol Crystallogr 55: 85-92
- PubMed: 10089398
- DOI: https://doi.org/10.1107/S0907444998007689
- Primary Citation of Related Structures:
1A43, 1BAJ - PubMed Abstract:
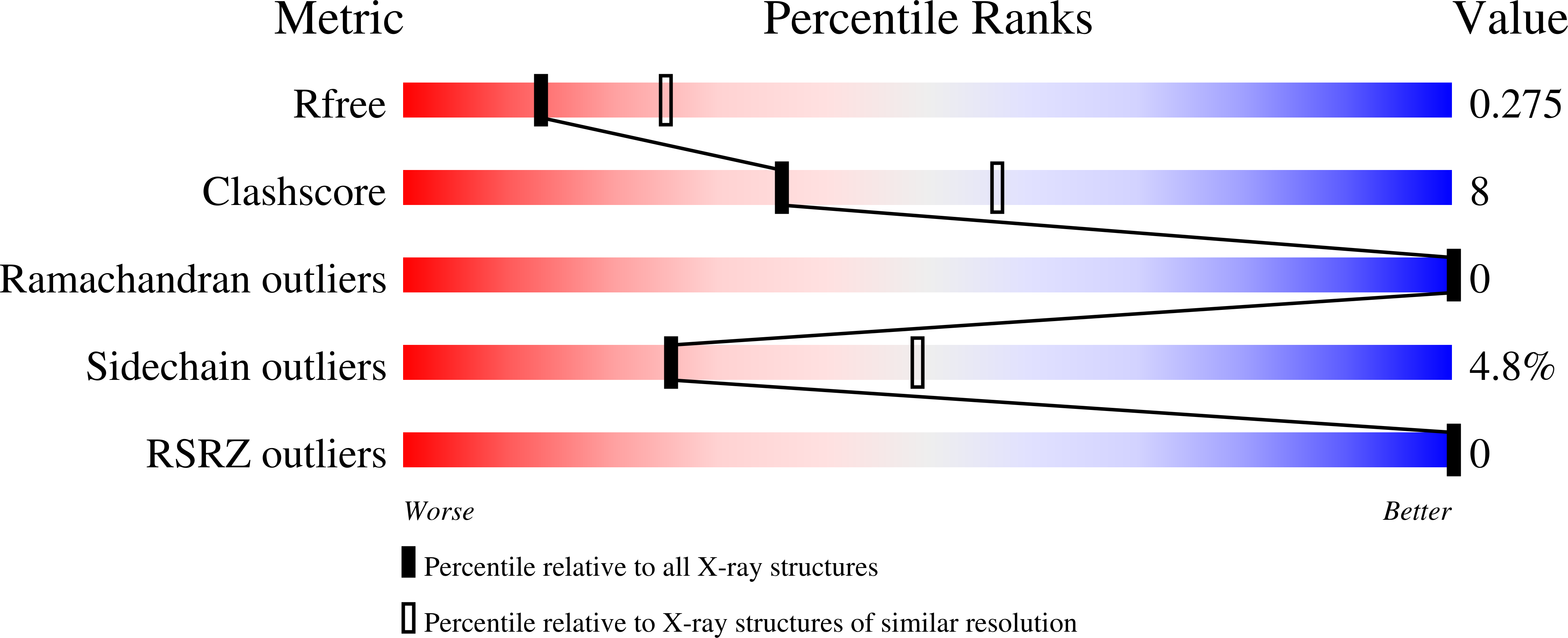
The human immunodeficiency virus type I (HIV-1) capsid protein is initially synthesized as the central domain of the Gag polyprotein, and is subsequently proteolytically processed into a discrete 231-amino-acid protein that forms the distinctive conical core of the mature virus. The crystal structures of two proteins that span the C-terminal domain of the capsid are reported here: one encompassing residues 146-231 (CA146-231) and the other extending to include the 14-residue p2 domain of Gag (CA146-p2). The isomorphous CA146-231 and CA146-p2 structures were determined by molecular replacement and have been refined at 2.6 A resolution to R factors of 22.3 and 20.7% (Rfree = 28.1 and 27.5%), respectively. The ordered domains comprise residues 148-219 for CA146-231 and 148-218 for CA146-p2, and their refined structures are essentially identical. The proteins are composed of a 310 helix followed by an extended strand and four alpha-helices. A crystallographic twofold generates a dimer that is stabilized by parallel packing of an alpha-helix 2 across the dimer interface and by packing of the 310 helix into a groove created by alpha-helices 2 and 3 of the partner molecule. CA146-231 and CA146-p2 dimerize with the full affinity of the intact capsid protein, and their structures therefore reveal the essential dimer interface of the HIV-1 capsid.
Organizational Affiliation:
Biochemistry Department, University of Utah Medical School, 50 North Medical Drive, Salt Lake City, Utah 84132, USA.