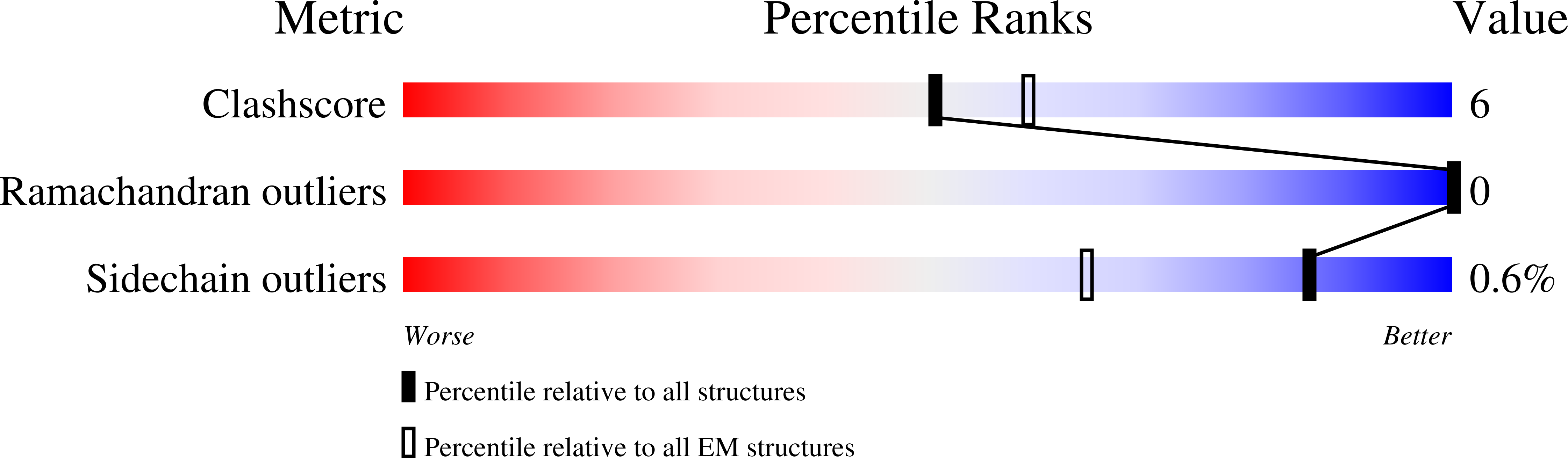Reply to: Acetyl-CoA is produced by the citrate synthase homology module of ATP-citrate lyase.
Wei, X., Marmorstein, R.(2021) Nat Struct Mol Biol 28: 639-641
- PubMed: 34294921
- DOI: https://doi.org/10.1038/s41594-021-00625-2
- Primary Citation of Related Structures:
7LIW, 7LJ9, 7LLA
Organizational Affiliation:
Department of Biochemistry & Biophysics, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA.