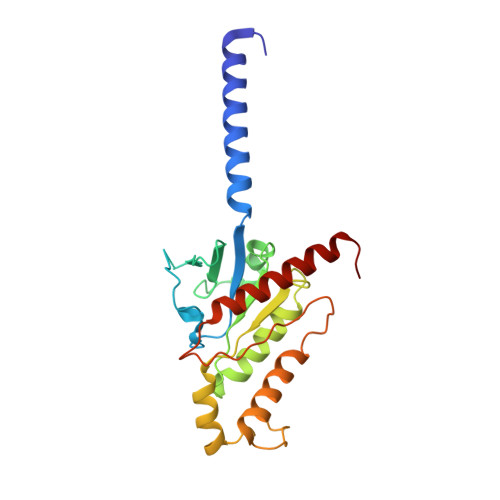
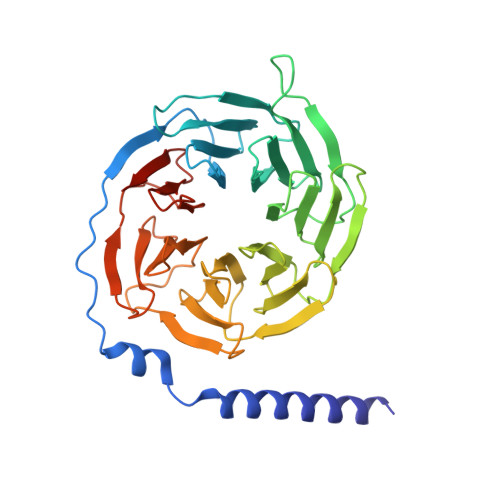
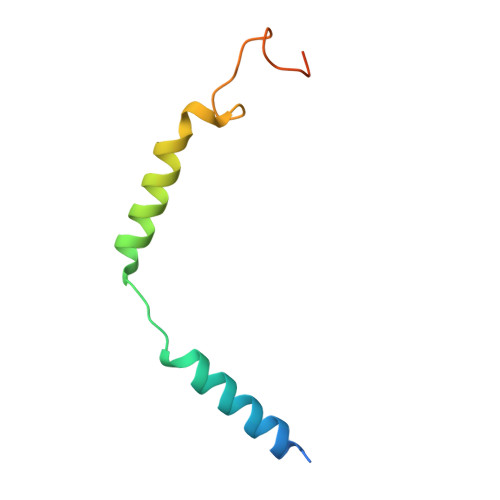
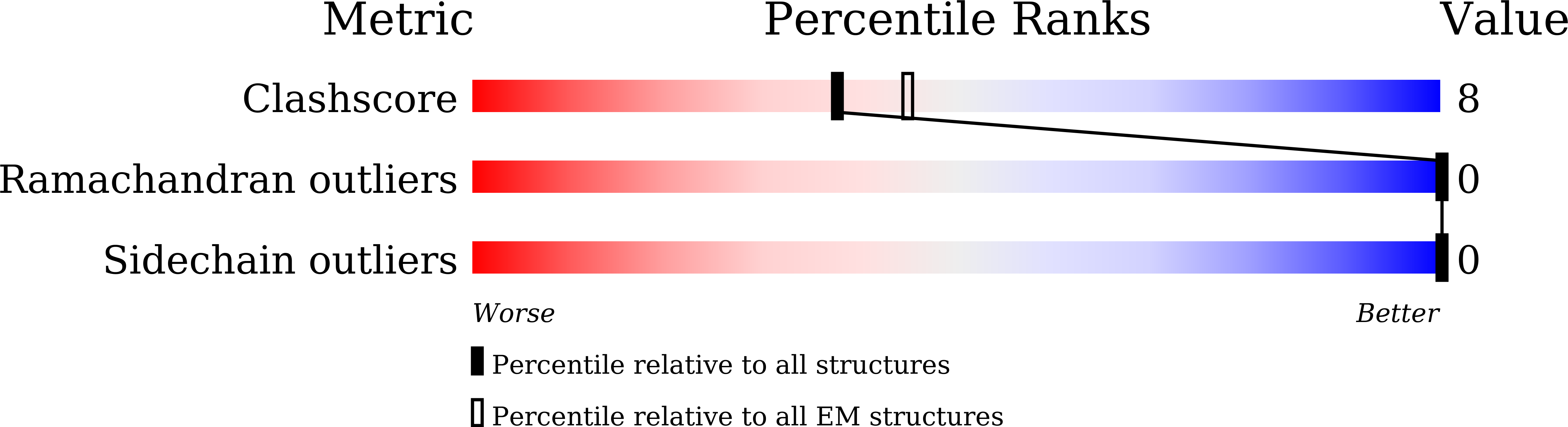Structures of the glucocorticoid-bound adhesion receptor GPR97-G o complex.
Ping, Y.Q., Mao, C., Xiao, P., Zhao, R.J., Jiang, Y., Yang, Z., An, W.T., Shen, D.D., Yang, F., Zhang, H., Qu, C., Shen, Q., Tian, C., Li, Z.J., Li, S., Wang, G.Y., Tao, X., Wen, X., Zhong, Y.N., Yang, J., Yi, F., Yu, X., Xu, H.E., Zhang, Y., Sun, J.P.(2021) Nature 589: 620-626
- PubMed: 33408414
- DOI: https://doi.org/10.1038/s41586-020-03083-w
- Primary Citation of Related Structures:
7D76, 7D77 - PubMed Abstract:
Adhesion G-protein-coupled receptors (GPCRs) are a major family of GPCRs, but limited knowledge of their ligand regulation or structure is available 1-3 . Here we report that glucocorticoid stress hormones activate adhesion G-protein-coupled receptor G3 (ADGRG3; also known as GPR97) 4-6 , a prototypical adhesion GPCR. The cryo-electron microscopy structures of GPR97-G o complexes bound to the anti-inflammatory drug beclomethasone or the steroid hormone cortisol revealed that glucocorticoids bind to a pocket within the transmembrane domain. The steroidal core of glucocorticoids is packed against the 'toggle switch' residue W 6.53 , which senses the binding of a ligand and induces activation of the receptor. Active GPR97 uses a quaternary core and HLY motif to fasten the seven-transmembrane bundle and to mediate G protein coupling. The cytoplasmic side of GPR97 has an open cavity, where all three intracellular loops interact with the G o protein, contributing to the high basal activity of GRP97. Palmitoylation at the cytosolic tail of the G o protein was found to be essential for efficient engagement with GPR97 but is not observed in other solved GPCR complex structures. Our work provides a structural basis for ligand binding to the seven-transmembrane domain of an adhesion GPCR and subsequent G protein coupling.
Organizational Affiliation:
CAS Key Laboratory of Receptor Research, Center for Structure and Function of Drug Targets, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai, China.