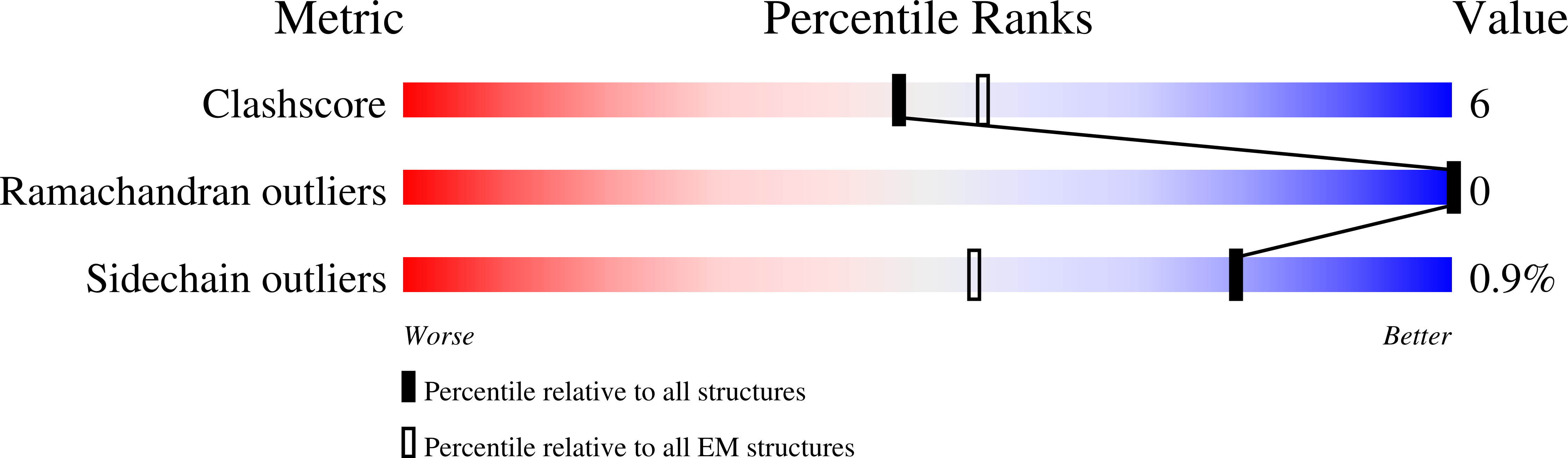Small-molecule-induced polymerization triggers degradation of BCL6.
Slabicki, M., Yoon, H., Koeppel, J., Nitsch, L., Roy Burman, S.S., Di Genua, C., Donovan, K.A., Sperling, A.S., Hunkeler, M., Tsai, J.M., Sharma, R., Guirguis, A., Zou, C., Chudasama, P., Gasser, J.A., Miller, P.G., Scholl, C., Frohling, S., Nowak, R.P., Fischer, E.S., Ebert, B.L.(2020) Nature 588: 164-168
- PubMed: 33208943
- DOI: https://doi.org/10.1038/s41586-020-2925-1
- Primary Citation of Related Structures:
6XMX - PubMed Abstract:
Effective and sustained inhibition of non-enzymatic oncogenic driver proteins is a major pharmacological challenge. The clinical success of thalidomide analogues demonstrates the therapeutic efficacy of drug-induced degradation of transcription factors and other cancer targets 1-3 , but a substantial subset of proteins are resistant to targeted degradation using existing approaches 4,5 . Here we report an alternative mechanism of targeted protein degradation, in which a small molecule induces the highly specific, reversible polymerization of a target protein, followed by its sequestration into cellular foci and subsequent degradation. BI-3802 is a small molecule that binds to the Broad-complex, Tramtrack and Bric-à-brac (BTB) domain of the oncogenic transcription factor B cell lymphoma 6 (BCL6) and leads to the proteasomal degradation of BCL6 6 . We use cryo-electron microscopy to reveal how the solvent-exposed moiety of a BCL6-binding molecule contributes to a composite ligand-protein surface that engages BCL6 homodimers to form a supramolecular structure. Drug-induced formation of BCL6 filaments facilitates ubiquitination by the SIAH1 E3 ubiquitin ligase. Our findings demonstrate that a small molecule such as BI-3802 can induce polymerization coupled to highly specific protein degradation, which in the case of BCL6 leads to increased pharmacological activity compared to the effects induced by other BCL6 inhibitors. These findings open new avenues for the development of therapeutic agents and synthetic biology.
Organizational Affiliation:
Broad Institute of MIT and Harvard, Cambridge, MA, USA.