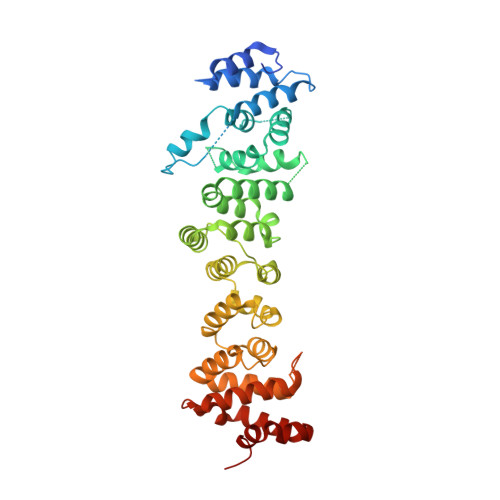
Structural basis for multifunctional roles of human Ints3 C-terminal domain.
Li, J., Ma, X., Banerjee, S., Baruah, S., Schnicker, N.J., Roh, E., Ma, W., Liu, K., Bode, A.M., Dong, Z.(2020) J Biol Chem 296: 100112-100112
- PubMed: 33434574
- DOI: https://doi.org/10.1074/jbc.RA120.016393
- Primary Citation of Related Structures:
6WLG - PubMed Abstract:
Proper repair of damaged DNA is critical for the maintenance of genome stability. A complex composed of Integrator subunit 3 (Ints3), single-stranded DNA-binding protein 1 (SSB1), and SSB-interacting protein 1 (SSBIP1) is required for efficient homologous recombination-dependent repair of double-strand breaks (DSBs) and ataxia-telangiectasia mutated (ATM)-dependent signaling pathways. It is known that in this complex the Ints3 N-terminal domain scaffolds SSB1 and SSBIP1. However, the molecular basis for the function of the Ints3 C-terminal domain remains unclear. Here, we present the crystal structure of the Ints3 C-terminal domain, uncovering a HEAT-repeat superhelical fold. Using structure and mutation analysis, we show that the C-terminal domain exists as a stable dimer. A basic groove and a cluster of conserved residues on two opposite sides of the dimer bind single-stranded RNA/DNA (ssRNA/ssDNA) and Integrator complex subunit 6 (Ints6), respectively. Dimerization is required for nucleic acid binding, but not for Ints6 binding. Additionally, in vitro experiments using HEK 293T cells demonstrate that Ints6 interaction is critical for maintaining SSB1 protein level. Taken together, our findings establish the structural basis of a multifunctional Ints3 C-terminal module, allowing us to propose a novel mode of nucleic acid recognition by helical repeat protein and paving the way for future mechanistic studies.
Organizational Affiliation:
The Hormel Institute, University of Minnesota, Austin, Minnesota, USA; China-US (Henan) Hormel Cancer Institute, Zhengzhou, Henan, China.