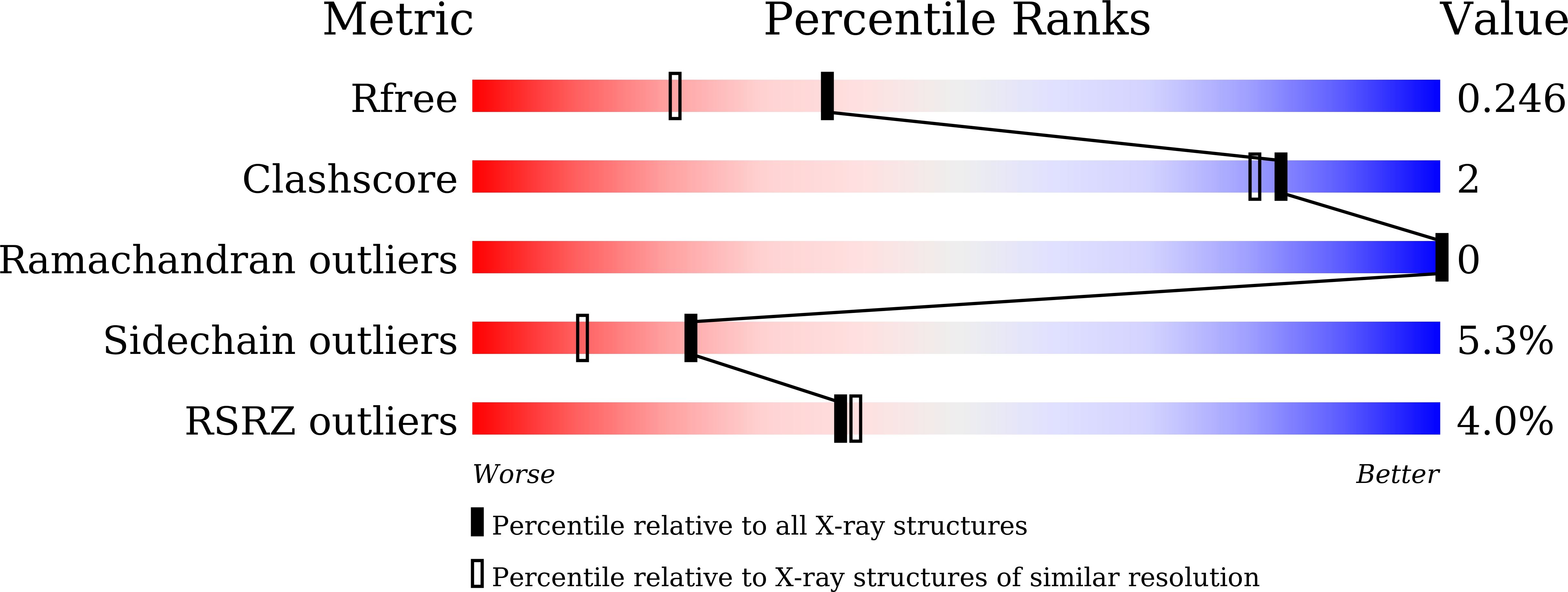
Dialysis-related amyloidosis associated with a novel beta 2 -microglobulin variant.
Mizuno, H., Hoshino, J., So, M., Kogure, Y., Fujii, T., Ubara, Y., Takaichi, K., Nakaniwa, T., Tanaka, H., Kurisu, G., Kametani, F., Nakagawa, M., Yoshinaga, T., Sekijima, Y., Higuchi, K., Goto, Y., Yazaki, M.(2021) Amyloid 28: 42-49
- PubMed: 32875920
- DOI: https://doi.org/10.1080/13506129.2020.1813097
- Primary Citation of Related Structures:
6M1B - PubMed Abstract:
Till date, there had been no reported case of dialysis-related amyloidosis (DRA) associated with a β 2 -microglobulin variant. We report here a 41-year-old haemodialysis patient with systemic amyloidosis, exhibiting macroglossia and swelling salivary glands, uncommon clinical manifestations for DRA. Molecular analysis showed that the patient had a new variant of β 2 -microglobulin (V27M). Extracted amyloid protein was predominantly composed of variant β 2 -microglobulin. In vitro analysis revealed that this variant β 2 -microglobulin had a strong amyloidogenic propensity, probably owing to the decreased stability caused by a bulky methionine residue. Our data clearly show that V27M variant is amyloidogenic and this mutation results in unusual clinical manifestations. To date, only one amyloidogenic β 2 -microglobulin variant (D76N) has been reported in non-dialysis patients. It is noteworthy that the V27M and D76N variants show substantial differences in both clinical phenotypes and pathomechanical features. This is the first case of DRA associated with a naturally occurring β 2 -microglobulin variant.
Organizational Affiliation:
Nephrology Center, Toranomon Hospital, Tokyo, Japan.