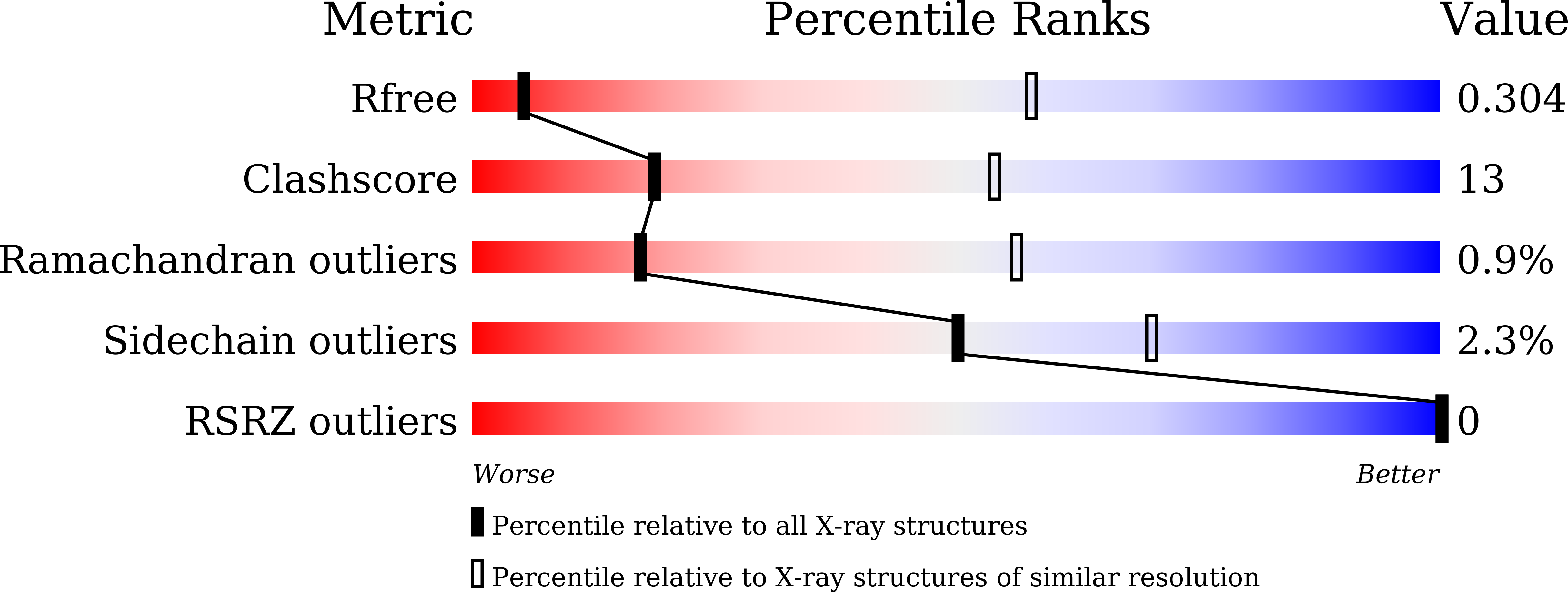
Blocking PPAR gamma interaction facilitates Nur77 interdiction of fatty acid uptake and suppresses breast cancer progression.
Yang, P.B., Hou, P.P., Liu, F.Y., Hong, W.B., Chen, H.Z., Sun, X.Y., Li, P., Zhang, Y., Ju, C.Y., Luo, L.J., Wu, S.F., Zhou, J.X., Wang, Z.J., He, J.P., Li, L., Zhao, T.J., Deng, X., Lin, T., Wu, Q.(2020) Proc Natl Acad Sci U S A 117: 27412-27422
- PubMed: 33087562
- DOI: https://doi.org/10.1073/pnas.2002997117
- Primary Citation of Related Structures:
6KZ5 - PubMed Abstract:
Nuclear receptor Nur77 participates in multiple metabolic regulations and plays paradoxical roles in tumorigeneses. Herein, we demonstrated that the knockout of Nur77 stimulated mammary tumor development in two mouse models, which would be reversed by a specific reexpression of Nur77 in mammary tissues. Mechanistically, Nur77 interacted and recruited corepressors, the SWI/SNF complex, to the promoters of CD36 and FABP4 to suppress their transcriptions, which hampered the fatty acid uptake, leading to the inhibition of cell proliferation. Peroxisome proliferator-activated receptor-γ (PPARγ) played an antagonistic role in this process through binding to Nur77 to facilitate ubiquitin ligase Trim13-mediated ubiquitination and degradation of Nur77. Cocrystallographic and functional analysis revealed that Csn-B, a Nur77-targeting compound, promoted the formation of Nur77 homodimer to prevent PPARγ binding by steric hindrance, thereby strengthening the Nur77's inhibitory role in breast cancer. Therefore, our study reveals a regulatory function of Nur77 in breast cancer via impeding fatty acid uptake.
Organizational Affiliation:
State Key Laboratory of Cellular Stress Biology, State-Province Joint Engineering Laboratory of Targeted Drugs from Natural Products, School of Life Sciences, Xiamen University, 361005 Xiamen, Fujian Province, China.