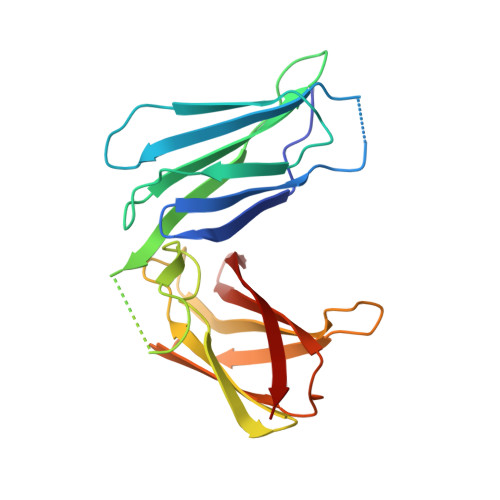
Integrin alpha 6 beta 4 Recognition of a Linear Motif of Bullous Pemphigoid Antigen BP230 Controls Its Recruitment to Hemidesmosomes.
Manso, J.A., Gomez-Hernandez, M., Carabias, A., Alonso-Garcia, N., Garcia-Rubio, I., Kreft, M., Sonnenberg, A., de Pereda, J.M.(2019) Structure 27: 952
- PubMed: 31006587
- DOI: https://doi.org/10.1016/j.str.2019.03.016
- Primary Citation of Related Structures:
6GVK, 6GVL - PubMed Abstract:
Mechanical stability of epithelia requires firm attachment to the basement membrane via hemidesmosomes. Dysfunction of hemidesmosomal proteins causes severe skin-blistering diseases. Two plakins, plectin and BP230 (BPAG1e), link the integrin α6β4 to intermediate filaments in epidermal hemidesmosomes. Here, we show that a linear sequence within the isoform-specific N-terminal region of BP230 binds to the third and fourth FnIII domains of β4. The crystal structure of the complex and mutagenesis analysis revealed that BP230 binds between the two domains of β4. BP230 induces closing of the two FnIII domains that are locked in place by an interdomain ionic clasp required for binding. Disruption of BP230-β4 binding prevents recruitment of BP230 to hemidesmosomes in human keratinocytes, revealing a key role of this interaction for hemidesmosome assembly. Phosphomimetic substitutions in β4 and BP230 destabilize the complex. Thus, our study provides insights into the architecture of hemidesmosomes and potential mechanisms of regulation.
Organizational Affiliation:
Instituto de Biología Molecular y Celular del Cáncer, Consejo Superior de Investigaciones Científicas - University of Salamanca, Campus Unamuno, 37007 Salamanca, Spain.