High-Resolution Structures for a Complex Between a Non-Helical Foldamer and VEGF
Thomas, N.C., London, H.S., Forest, K.T., Gellman, S.H.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Vascular endothelial growth factor A | 102 | Homo sapiens | Mutation(s): 0 Gene Names: VEGFA, VEGF | 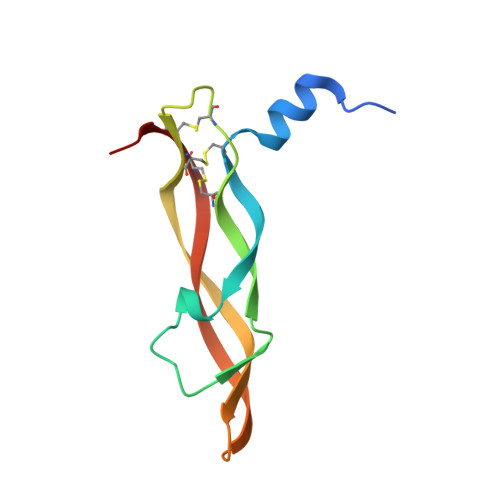 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P15692 (Homo sapiens) Explore P15692 Go to UniProtKB: P15692 | |||||
PHAROS: P15692 GTEx: ENSG00000112715 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P15692 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Vascular endothelial growth factor A | 102 | Homo sapiens | Mutation(s): 0 Gene Names: VEGFA, VEGF | 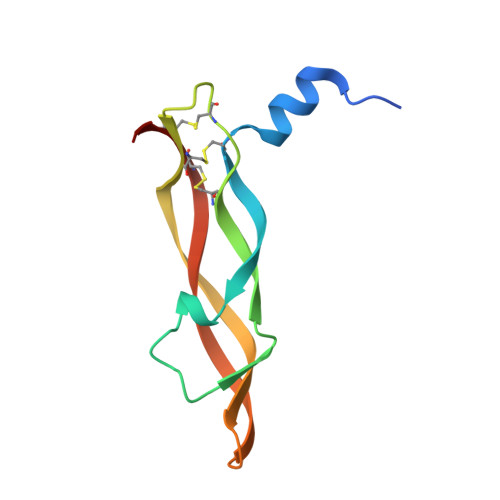 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P15692 (Homo sapiens) Explore P15692 Go to UniProtKB: P15692 | |||||
PHAROS: P15692 GTEx: ENSG00000112715 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P15692 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Find similar proteins by: Sequence | 3D Structure
Entity ID: 3 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| HH4 alpha/beta-Peptide | 15 | synthetic construct | Mutation(s): 0 | 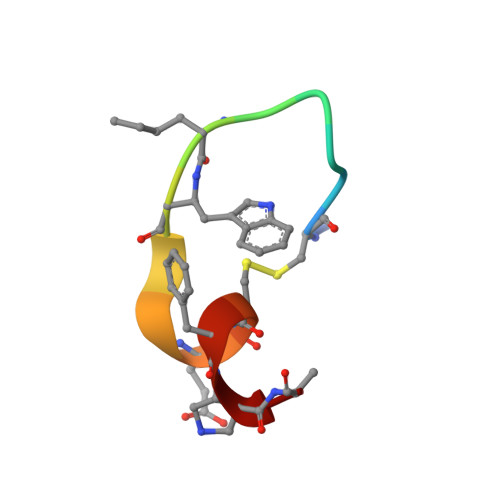 | |
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Modified Residues 3 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Type | Formula | 2D Diagram | Parent |
| B3E Query on B3E | C, D | L-PEPTIDE LINKING | C6 H11 N O4 |  | GLU |
| HT7 Query on HT7 | C, D | L-PEPTIDE LINKING | C12 H14 N2 O2 |  | TRP |
| NLE Query on NLE | C, D | L-PEPTIDE LINKING | C6 H13 N O2 |  | LEU |
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 101.649 | α = 90 |
| b = 101.649 | β = 90 |
| c = 59.988 | γ = 120 |
| Software Name | Purpose |
|---|---|
| REFMAC | refinement |
| XDS | data reduction |
| SCALA | data scaling |
| PHASER | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| National Institutes of Health/National Institute of General Medical Sciences (NIH/NIGMS) | United States | GM056414 |