Reviving B-Factors: Activating ALK Mutations Increase Protein Dynamics of the Unphosphorylated Kinase.
Johnson, T.W., Bolanos, B., Brooun, A., Gallego, R.A., Gehlhaar, D., Jalaie, M., McTigue, M., Timofeevski, S.(2018) ACS Med Chem Lett 9: 872-877
- PubMed: 30258533
- DOI: https://doi.org/10.1021/acsmedchemlett.8b00348
- Primary Citation of Related Structures:
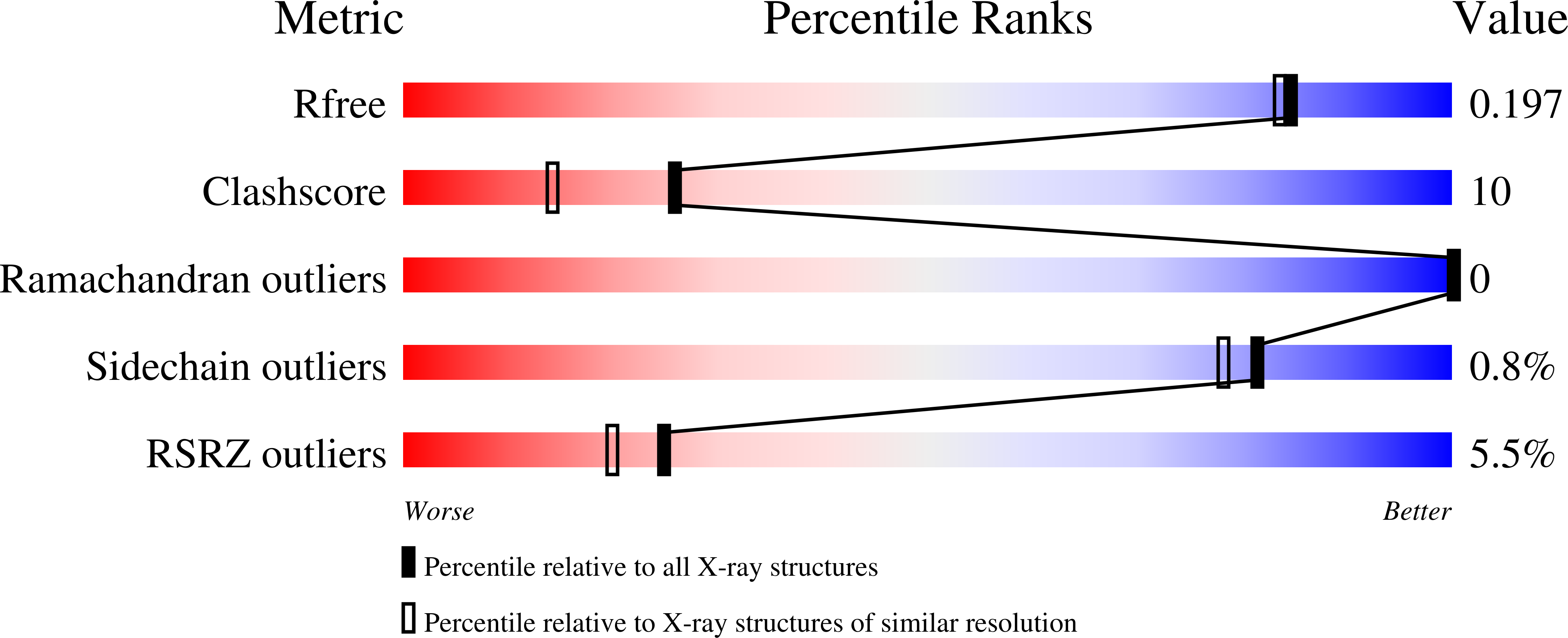
6CDT - PubMed Abstract:
Anaplastic lymphoma kinase (ALK) is a receptor tyrosine kinase that can become oncogenic by activating mutations or overexpression. Full kinetic characterization of both phosphorylated and nonphosphorylated wildtype and mutant ALK kinase domain was done. Our structure-based drug design programs directed at ALK allowed us to interrogate whether X-ray crystallography data could be used to support the hypothesis that activation of ALK by mutation occurs due to increased protein dynamics. Crystallographic B-factors were converted to normalized B-factors, which allowed analysis of wildtype ALK, ALK-C1156Y, and ALK-L1196M. This data suggests that mobility of the P-loop, αC-helix, and activation loop (A-loop) may be important in catalytic activity increases, with or without phosphorylation. Both molecular dynamics simulations and hydrogen-deuterium exchange experimental data corroborated the normalized B-factors data.
Organizational Affiliation:
Pfizer Worldwide Research and Development, La Jolla Oncology, 10770 Science Center Drive, San Diego, California 92121, United States.