Hysteresis and Allostery in Human UDP-Glucose Dehydrogenase Require a Flexible Protein Core.
Beattie, N.R., Pioso, B.J., Sidlo, A.M., Keul, N.D., Wood, Z.A.(2018) Biochemistry 57: 6848-6859
- PubMed: 30457329
- DOI: https://doi.org/10.1021/acs.biochem.8b00497
- Primary Citation of Related Structures:
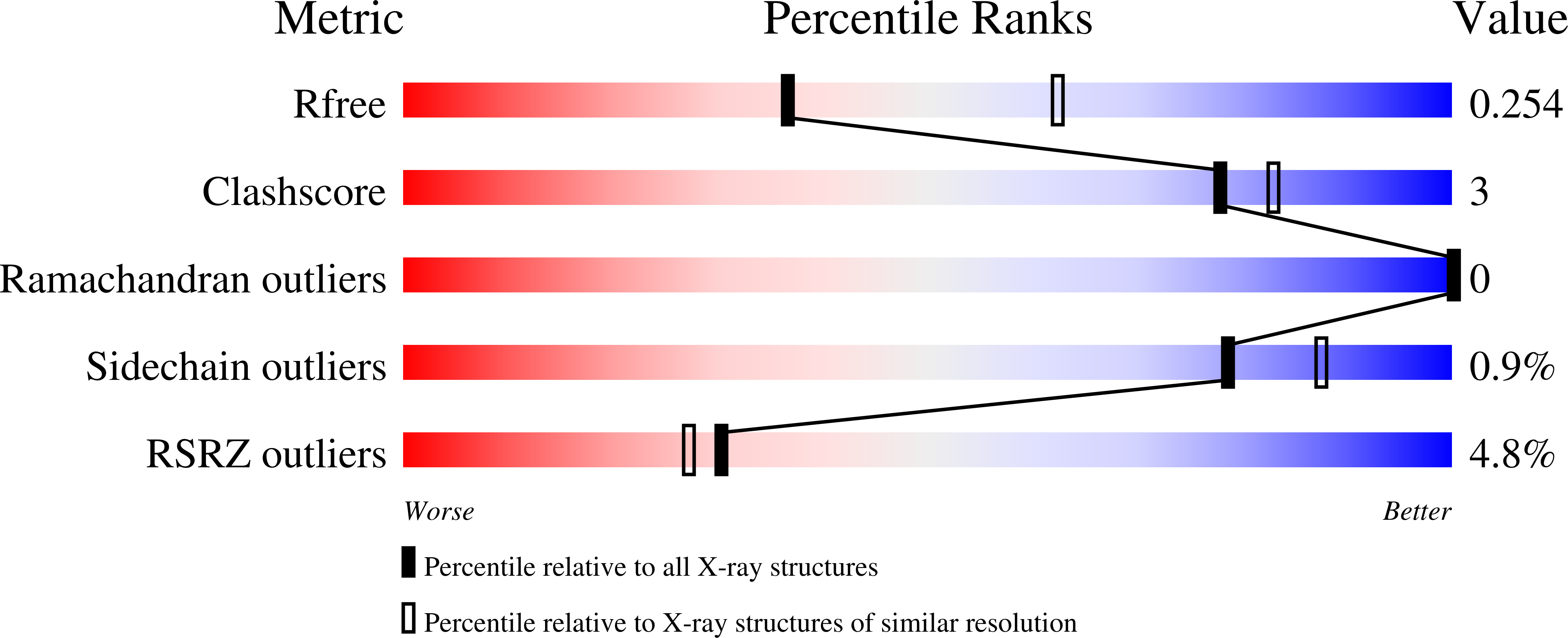
6C4J, 6C4K - PubMed Abstract:
Human UDP-glucose dehydrogenase (hUGDH) oxidizes UDP-glucose to UDP-glucuronic acid, an essential substrate in the phase II metabolism of drugs. The activity of hUGDH is regulated by the conformation of a buried allosteric switch (T131 loop/α6 helix). Substrate binding induces the allosteric switch to slowly isomerize from an inactive E* conformation to the active E state, which can be observed as enzyme hysteresis. When the feedback inhibitor UDP-xylose binds, the allosteric switch and surrounding residues in the protein core repack, converting the hexamer into an inactive, horseshoe-shaped complex (E Ω ). This allosteric transition is facilitated by large cavities and declivities in the protein core that provide the space required to accommodate the alternate packing arrangements. Here, we have used the A104L substitution to fill a cavity in the E state and sterically prevent repacking of the core into the E Ω state. Steady state analysis shows that hUGDH A104L binds UDP-xylose with lower affinity and that the inhibition is no longer cooperative. This means that the allosteric transition to the high-UDP-xylose affinity E Ω state is blocked by the substitution. The crystal structures of hUGDH A104L show that the allosteric switch still adopts the E and E* states, albeit with a more rigid protein core. However, the progress curves of hUGDH A104L do not show hysteresis, which suggests that the E* and E states are now in rapid equilibrium. Our data suggest that hysteresis in native hUGDH originates from the conformational entropy of the E* state protein core.
Organizational Affiliation:
Department of Biochemistry & Molecular Biology , University of Georgia , Athens , Georgia 30602 , United States.