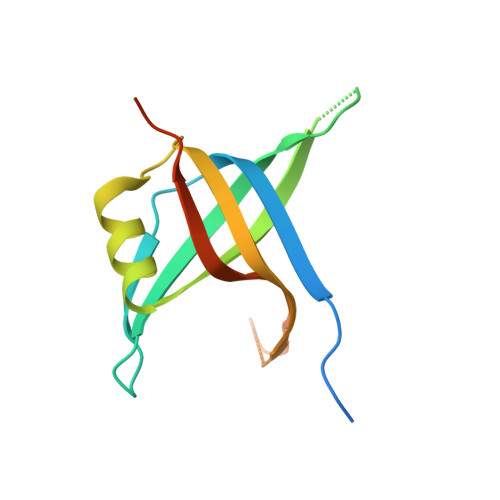
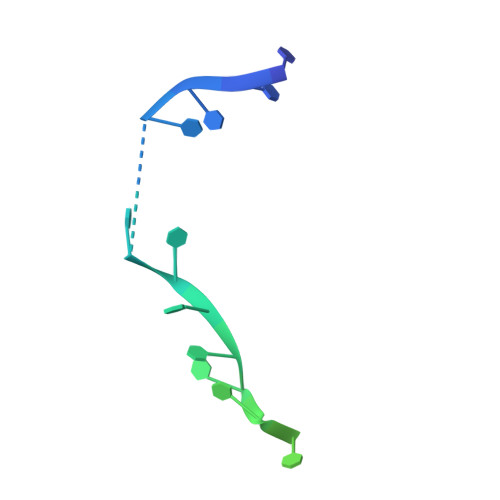
Structural Mechanisms of Cooperative DNA Binding by Bacterial Single-Stranded DNA-Binding Proteins.
Dubiel, K., Myers, A.R., Kozlov, A.G., Yang, O., Zhang, J., Ha, T., Lohman, T.M., Keck, J.L.(2019) J Mol Biol 431: 178-195
- PubMed: 30472092
- DOI: https://doi.org/10.1016/j.jmb.2018.11.019
- Primary Citation of Related Structures:
6BHW, 6BHX - PubMed Abstract:
Bacteria encode homooligomeric single-stranded (ss) DNA-binding proteins (SSBs) that coat and protect ssDNA intermediates formed during genome maintenance reactions. The prototypical Escherichia coli SSB tetramer can bind ssDNA using multiple modes that differ by the number of bases bound per tetramer and the magnitude of the binding cooperativity. Our understanding of the mechanisms underlying cooperative ssDNA binding by SSBs has been hampered by the limited amount of structural information available for interfaces that link adjacent SSB proteins on ssDNA. Here we present a crystal structure of Bacillus subtilis SsbA bound to ssDNA. The structure resolves SsbA tetramers joined together by a ssDNA "bridge" and identifies an interface, termed the "bridge interface," that links adjacent SSB tetramers through an evolutionarily conserved surface near the ssDNA-binding site. E. coli SSB variants with altered bridge interface residues bind ssDNA with reduced cooperativity and with an altered distribution of DNA binding modes. These variants are also more readily displaced from ssDNA by RecA than wild-type SSB. In spite of these biochemical differences, each variant is able to complement deletion of the ssb gene in E. coli. Together our data suggest a model in which the bridge interface contributes to cooperative ssDNA binding and SSB function but that destabilization of the bridge interface is tolerated in cells.
Organizational Affiliation:
Department of Biomolecular Chemistry, University of Wisconsin School of Medicine and Public Health, Madison, WI 53706, USA.