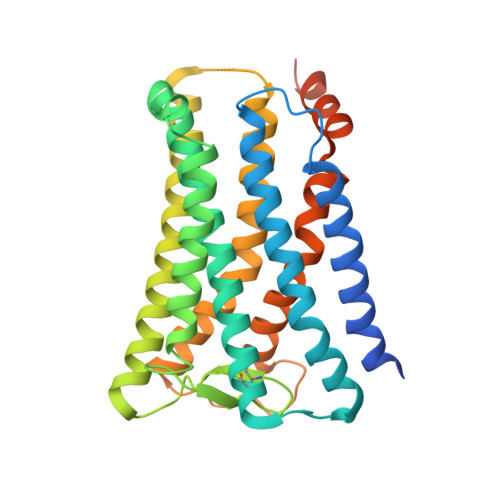
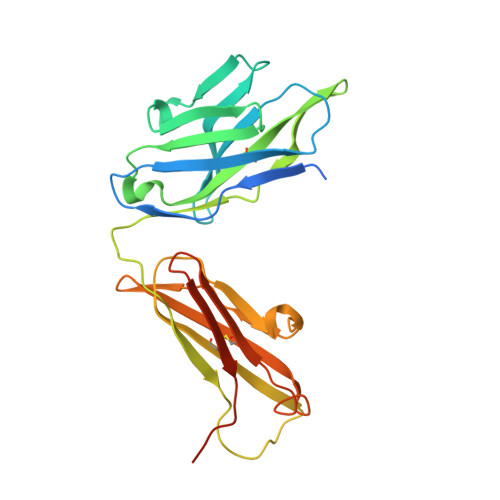
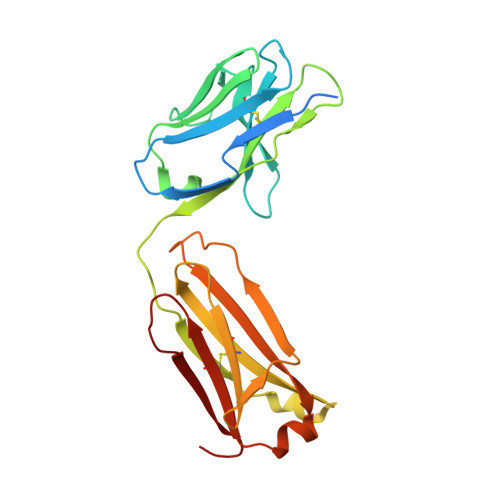
Ligand binding to human prostaglandin E receptor EP4at the lipid-bilayer interface.
Toyoda, Y., Morimoto, K., Suno, R., Horita, S., Yamashita, K., Hirata, K., Sekiguchi, Y., Yasuda, S., Shiroishi, M., Shimizu, T., Urushibata, Y., Kajiwara, Y., Inazumi, T., Hotta, Y., Asada, H., Nakane, T., Shiimura, Y., Nakagita, T., Tsuge, K., Yoshida, S., Kuribara, T., Hosoya, T., Sugimoto, Y., Nomura, N., Sato, M., Hirokawa, T., Kinoshita, M., Murata, T., Takayama, K., Yamamoto, M., Narumiya, S., Iwata, S., Kobayashi, T.(2019) Nat Chem Biol 15: 18-26
- PubMed: 30510193
- DOI: https://doi.org/10.1038/s41589-018-0131-3
- Primary Citation of Related Structures:
5YFI, 5YHL, 5YWY - PubMed Abstract:
Prostaglandin E receptor EP4, a G-protein-coupled receptor, is involved in disorders such as cancer and autoimmune disease. Here, we report the crystal structure of human EP4 in complex with its antagonist ONO-AE3-208 and an inhibitory antibody at 3.2 Å resolution. The structure reveals that the extracellular surface is occluded by the extracellular loops and that the antagonist lies at the interface with the lipid bilayer, proximal to the highly conserved Arg316 residue in the seventh transmembrane domain. Functional and docking studies demonstrate that the natural agonist PGE 2 binds in a similar manner. This structural information also provides insight into the ligand entry pathway from the membrane bilayer to the EP4 binding pocket. Furthermore, the structure reveals that the antibody allosterically affects the ligand binding of EP4. These results should facilitate the design of new therapeutic drugs targeting both orthosteric and allosteric sites in this receptor family.
Organizational Affiliation:
Department of Medical Chemistry and Cell Biology, Kyoto University Graduate School of Medicine, Konoe-cho, Yoshida, Sakyo-ku, Kyoto, Japan.