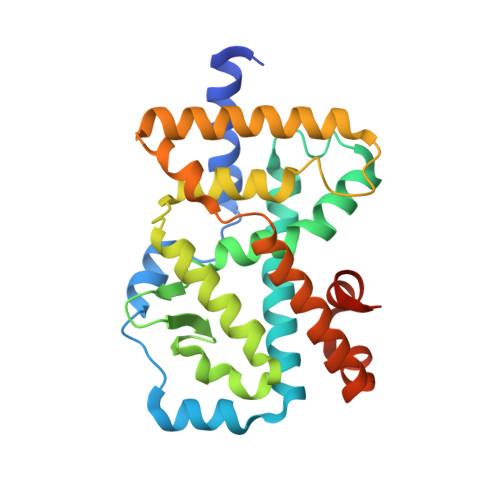
Structural determinant for inducing RORgamma specific inverse agonism triggered by a synthetic benzoxazinone ligand.
Marcotte, D.J., Liu, Y., Little, K., Jones, J.H., Powell, N.A., Wildes, C.P., Silvian, L.F., Chodaparambil, J.V.(2016) BMC Struct Biol 16: 7-7
- PubMed: 27246200
- DOI: https://doi.org/10.1186/s12900-016-0059-3
- Primary Citation of Related Structures:
5IXK, 5IZ0 - PubMed Abstract:
The nuclear hormone receptor RORγ regulates transcriptional genes involved in the production of the pro-inflammatory interleukin IL-17 which has been linked to autoimmune diseases such as rheumatoid arthritis, multiple sclerosis and inflammatory bowel disease. This transcriptional activity of RORγ is modulated through a protein-protein interaction involving the activation function 2 (AF2) helix on the ligand binding domain of RORγ and a conserved LXXLL helix motif on coactivator proteins. Our goal was to develop a RORγ specific inverse agonist that would help down regulate pro-inflammatory gene transcription by disrupting the protein protein interaction with coactivator proteins as a therapeutic agent. We identified a novel series of synthetic benzoxazinone ligands having an agonist (BIO592) and inverse agonist (BIO399) mode of action in a FRET based assay. We show that the AF2 helix of RORγ is proteolytically sensitive when inverse agonist BIO399 binds. Using x-ray crystallography we show how small modifications on the benzoxazinone agonist BIO592 trigger inverse agonism of RORγ. Using an in vivo reporter assay, we show that the inverse agonist BIO399 displayed specificity for RORγ over ROR sub-family members α and β. The synthetic benzoxazinone ligands identified in our FRET assay have an agonist (BIO592) or inverse agonist (BIO399) effect by stabilizing or destabilizing the agonist conformation of RORγ. The proteolytic sensitivity of the AF2 helix of RORγ demonstrates that it destabilizes upon BIO399 inverse agonist binding perturbing the coactivator protein binding site. Our structural investigation of the BIO592 agonist and BIO399 inverse agonist structures identified residue Met358 on RORγ as the trigger for RORγ specific inverse agonism.
Organizational Affiliation:
Chemical and Molecular Therapeutics, Biogen Inc, 250 Binney Street, Cambridge, MA, 02142, USA. doug.marcotte@biogenidec.com.