Structure of human neuropilin-2 b1 domain with novel and unique zinc binding site
Tsai, Y.I., Frankel, P., Rana, R.R., Fotinou, C., Yelland, T.S., Zachary, I., Djordjevic, S.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Neuropilin-2 | 162 | Homo sapiens | Mutation(s): 0 Gene Names: NRP2, VEGF165R2 | 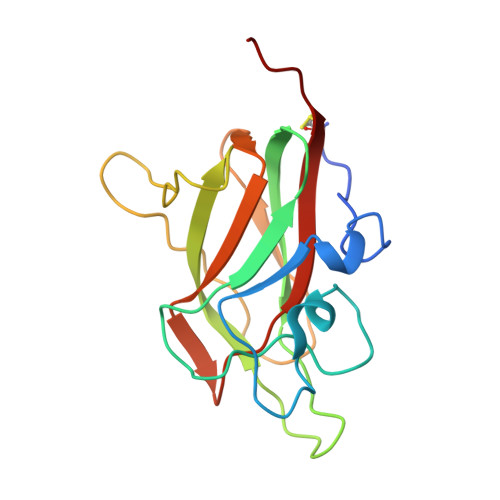 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for O60462 (Homo sapiens) Explore O60462 Go to UniProtKB: O60462 | |||||
PHAROS: O60462 GTEx: ENSG00000118257 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | O60462 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 4 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| MES Query on MES | I [auth A] | 2-(N-MORPHOLINO)-ETHANESULFONIC ACID C6 H13 N O4 S SXGZJKUKBWWHRA-UHFFFAOYSA-N |  | ||
| ZN Query on ZN | B [auth A], C [auth A], D [auth A] | ZINC ION Zn PTFCDOFLOPIGGS-UHFFFAOYSA-N |  | ||
| EDO Query on EDO | G [auth A], H [auth A] | 1,2-ETHANEDIOL C2 H6 O2 LYCAIKOWRPUZTN-UHFFFAOYSA-N |  | ||
| CL Query on CL | E [auth A], F [auth A] | CHLORIDE ION Cl VEXZGXHMUGYJMC-UHFFFAOYSA-M |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 99.39 | α = 90 |
| b = 105.27 | β = 90 |
| c = 45.75 | γ = 90 |
| Software Name | Purpose |
|---|---|
| REFMAC | refinement |
| Coot | model building |
| SCALA | data scaling |
| MOSFLM | data reduction |
| PHASER | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| British Heart Foundation | United Kingdom | PG/10/52/28448 |