Crystal Structures of a GH131 beta-Glucanase Catalytic Domain from Podospora anserina in Complex with Cellotriose
Jiang, T., Chan, H.C., Huang, C.H., Ko, T.P., Huang, T.Y., Liu, J.R., Guo, R.T.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Beta-glucanase | 252 | Podospora anserina | Mutation(s): 0 | 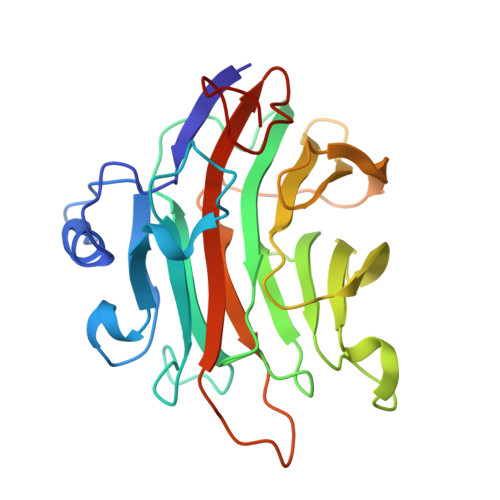 | |
UniProt | |||||
Find proteins for J7K096 (Podospora anserina) Explore J7K096 Go to UniProtKB: J7K096 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | J7K096 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| ID | Chains | Name | Type/Class | 2D Diagram | 3D Interactions |
| PRD_900021 Query on PRD_900021 | E | beta-cellotriose | Oligosaccharide / Metabolism |  | |
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 54.623 | α = 81.54 |
| b = 61.854 | β = 75.16 |
| c = 79.041 | γ = 77.27 |
| Software Name | Purpose |
|---|---|
| HKL-2000 | data collection |
| PHASER | phasing |
| CNS | refinement |
| HKL-2000 | data reduction |
| HKL-2000 | data scaling |