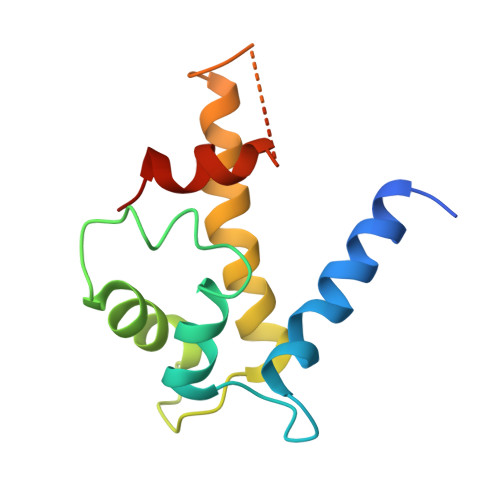
Structure of an asymmetric ternary protein complex provides insight for membrane interaction.
Dempsey, B.R., Rezvanpour, A., Lee, T.W., Barber, K.R., Junop, M.S., Shaw, G.S.(2012) Structure 20: 1737-1745
- PubMed: 22940583
- DOI: https://doi.org/10.1016/j.str.2012.08.004
- Primary Citation of Related Structures:
4DRW - PubMed Abstract:
Plasma membrane repair involves the coordinated effort of proteins and the inner phospholipid surface to mend the rupture and return the cell back to homeostasis. Here, we present the three-dimensional structure of a multiprotein complex that includes S100A10, annexin A2, and AHNAK, which along with dysferlin, functions in muscle and cardiac tissue repair. The 3.5 Å resolution X-ray structure shows that a single region from the AHNAK C terminus is recruited by an S100A10-annexin A2 heterotetramer, forming an asymmetric ternary complex. The AHNAK peptide adopts a coil conformation that arches across the heterotetramer contacting both annexin A2 and S100A10 protomers with tight affinity (∼30 nM) and establishing a structural rationale whereby both S100A10 and annexin proteins are needed in AHNAK recruitment. The structure evokes a model whereby AHNAK is targeted to the membrane surface through sandwiching of the binding region between the S100A10/annexin A2 complex and the phospholipid membrane.
Organizational Affiliation:
Department of Biochemistry, The University of Western Ontario, London, Ontario N6A 5C1, Canada.